Cyclophosphamide monohydrate |
| Catalog No.GC14077 |
An alkylating agent
Products are for research use only. Not for human use. We do not sell to patients.
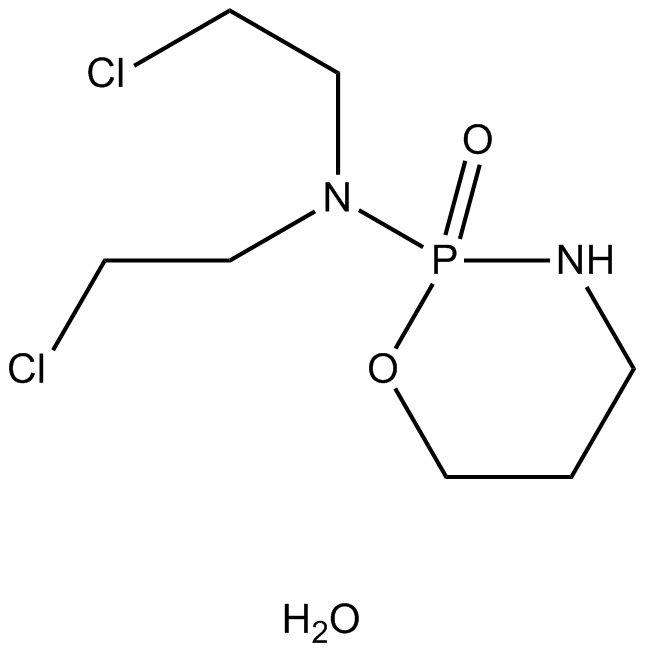
Cas No.: 6055-19-2
Sample solution is provided at 25 µL, 10mM.
IC50: 511 μM for AChE
Cyclophosphamide monohydrate is used in the cancer treatment in children. As a prodrug it should be metabolized by cytochrome P-450 enzymes to produce the active alkylating species, which are responsible for its cytotoxic effects.
In vitro: Cyclophosphamide inhibited the AChE reversibly with an IC50 of 511 μM. In the control system, Km was 132 μM for AChE, which increased by 78% in the CP treated system. The nature of cyclophosphamide was of the linear mixed type (partially competitive and purely noncompetitive). The values of Ki and KI were estimated to be 378 and 582 μM respectively, indicating that noncompetitive inhibition was predominant over competitive [1].
In vivo: PK studies in mice revealed a delayed plasma clearance of cyclophosphamide after carbon-tetrachloride pretreatment. Plasma levels of total alkylating activity and 4-hydroxycyclophosphamide increased more slowly and reached a lower peak. However, there was no difference in the AUC for either plasma total alkylating activity or plasma 4-hydroxycyclophosphamide between two groups. Thus, prolonged exposure of tumor cells to 4-hydroxycyclophosphamide might be responsible for the increased antitumor activity of cyclophosphamide following carbon-tetrachloride pretreatment [2].
Clinical trial: Twenty-two children not receiving other therapy known to influence drug metabolism were selected, of whom, nine were receiving combination treatment and thirteen were identified as controls. Results showed that the the plasma cyclophosphamide clearance was lower in patients receiving fluconazole combined group [3].
References:
[1] al-Jafari AA,Duhaiman AS,Kamal MA. Inhibition of human acetylcholinesterase by cyclophosphamide. Toxicology.1995 Jan 19;96(1):1-6.
[2] Harris RN,Basseches PJ,Appel PL,Durski AM,Powis G. Carbon tetrachloride-induced increase in the antitumor activity of cyclophosphamide in mice: a pharmacokinetic study. Cancer Chemother Pharmacol.1984;12(3):167-72.
[3] Yule SM,Walker D,Cole M,McSorley L,Cholerton S,Daly AK,Pearson AD,Boddy AV. The effect of fluconazole on cyclophosphamide metabolism in children. Drug Metab Dispos.1999 Mar;27(3):417-21.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




