DNA Alkylator/Crosslinker
DNA alkylator/crosslinker is a molecule that alkylates DNA or can cross link with DNA. DNA alkylator/crosslinker can have mutagenic, pharmaceutical, or other effects. Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene. Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in organic molecules. Selective alkylation, or adding parts to the chain with the desired functional groups, is used, especially if there is no commonly available biological precursor. Alkylation with only one carbon is termed methylation. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Crosslinking of DNA occurs when various exogenous or endogenous agents react with two different positions in the DNA. This can either occur in the same strand (intrastrand crosslink) or in the opposite strands of the DNA (interstrand crosslink). Crosslinks also occur between DNA and protein. DNA replication is blocked by crosslinks, which causes replication arrest and cell death if the crosslink is not repaired. The RAD51 family plays a role in repair.
Targets for DNA Alkylator/Crosslinker
Products for DNA Alkylator/Crosslinker
- Cat.No. Product Name Information
-
GC34955
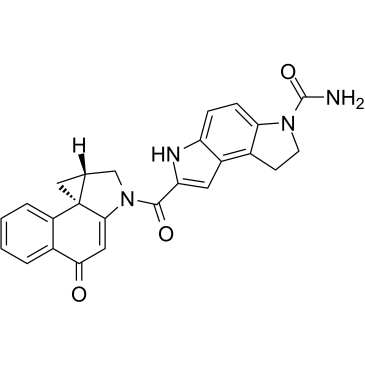
(+)-CBI-CDPI1
(+)-CBI-CDPI1 is an enhanced functional analog of CC-1065. (+)-CBI-CDPI1 is a DNA alkylating agent. (+)-CBI-CDPI1 is an antibody drug conjugates (ADCs) toxin.
-
GC34956
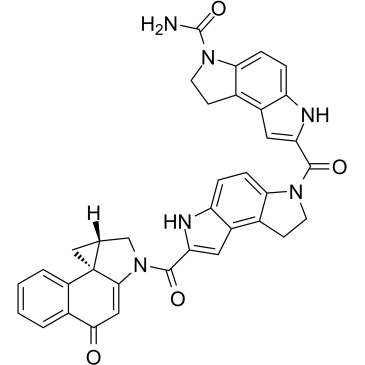
(+)-CBI-CDPI2
(+)-CBI-CDPI2 is an enhanced functional analog of CC-1065. (+)-CBI-CDPI1 is a DNA alkylating agent. (+)-CBI-CDPI2 is an antibody drug conjugates (ADCs) toxin.
-
GC60421
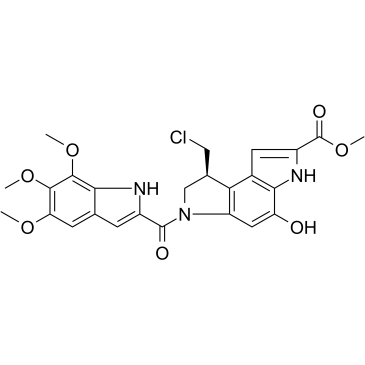
(S)-Seco-Duocarmycin SA
(S)-Seco-Duocarmycin SA is a DNA alkylator, cytotoxic to cancer cells, and acts as a ADC cytotoxin for antibody-drug conjugates.
-
GC49470
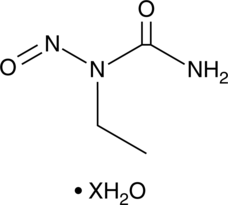
1-Ethyl-1-nitrosourea (hydrate)
A DNA alkylating agent
-
GC52129
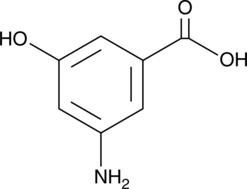
3-Amino-5-hydroxybenzoic Acid
-
GC42401
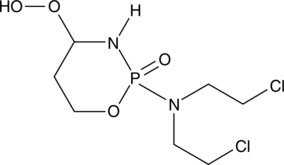
4-hydroperoxy Cyclophosphamide
An activated analog of cyclophosphamide
-
GC68332
4-Hydroperoxy Cyclophosphamide-d4

-
GC14564
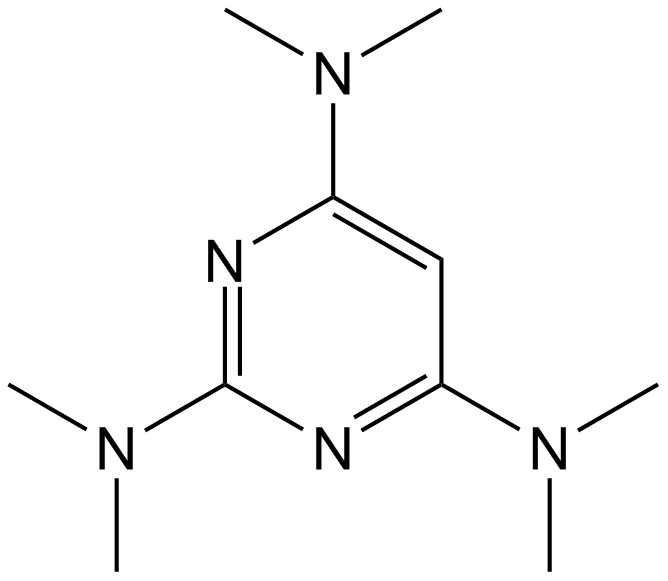
Altretamine
Antineoplastic agent
-
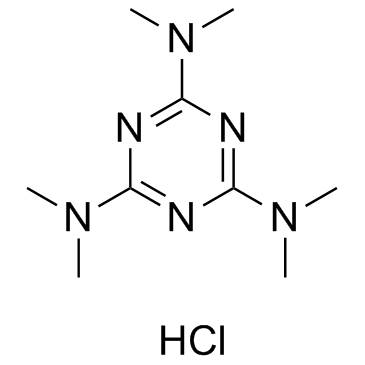
GC35310
Altretamine hydrochloride
Altretamine hydrochloride is an alkylating antineoplastic agent.
-
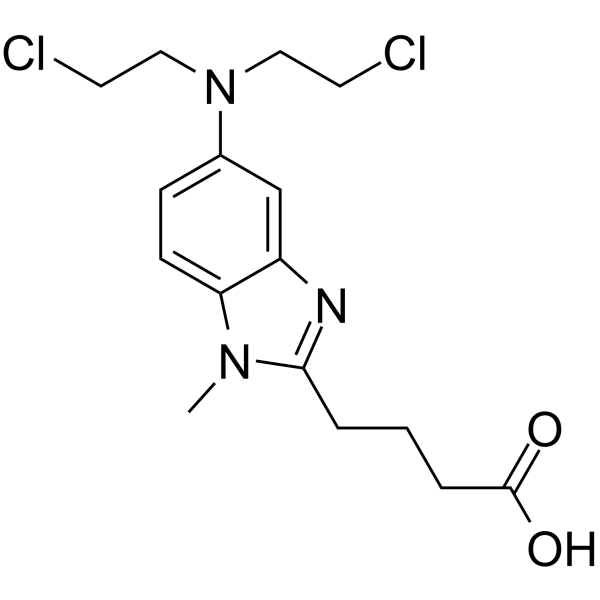
GC64354
Bendamustine
Bendamustine (SDX-105 free base), a purine analogue, is a DNA cross-linking agent. Bendamustine activates DNA-damage stress response and apoptosis. Bendamustine has potent alkylating, anticancer and antimetabolite properties.
-
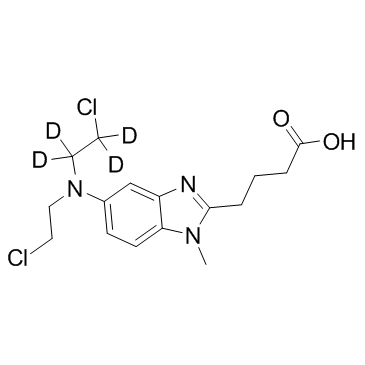
GC34305
Bendamustine D4 (SDX-105 D4)
-
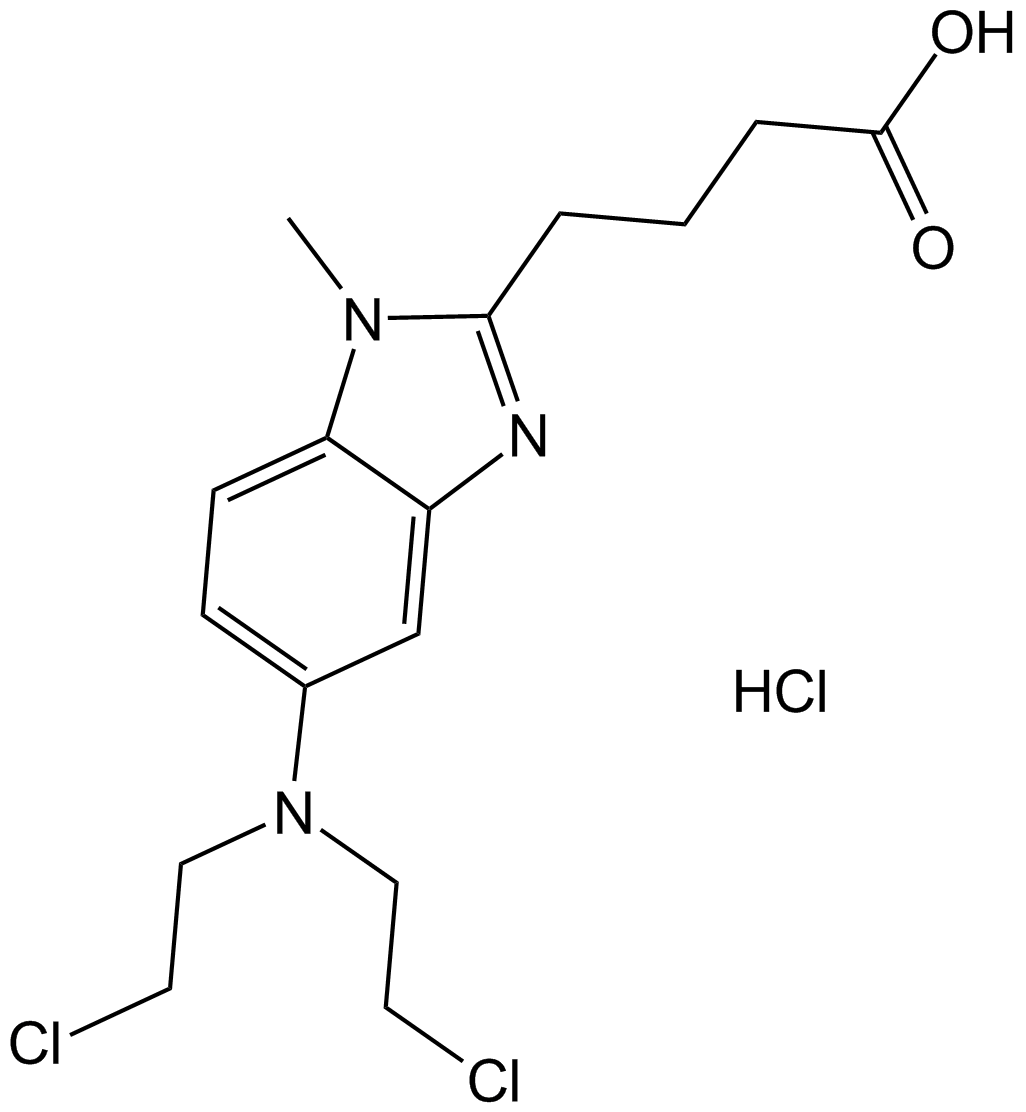
GC10744
Bendamustine HCl
Bendamustine HCl (SDX-105), a purine analogue, is a DNA cross-linking agent. Bendamustine HCl activats DNA-damage stress response and apoptosis. Bendamustine HCl has potent alkylating, anticancer and antimetabolite properties.
-
GC13671
Busulfan
DNA alkylating agent
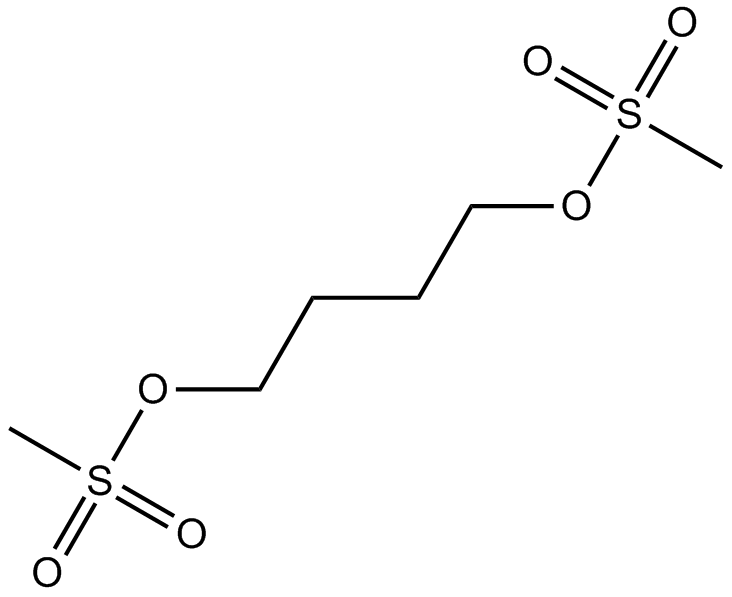
-
GC46962
Busulfan-d8
An internal standard for the quantification of busulfan
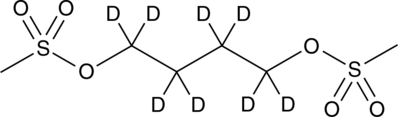
-
GC19086
Calicheamicin
Calicheamicin is a cytotoxic agent that causes double-strand DNA breaks.
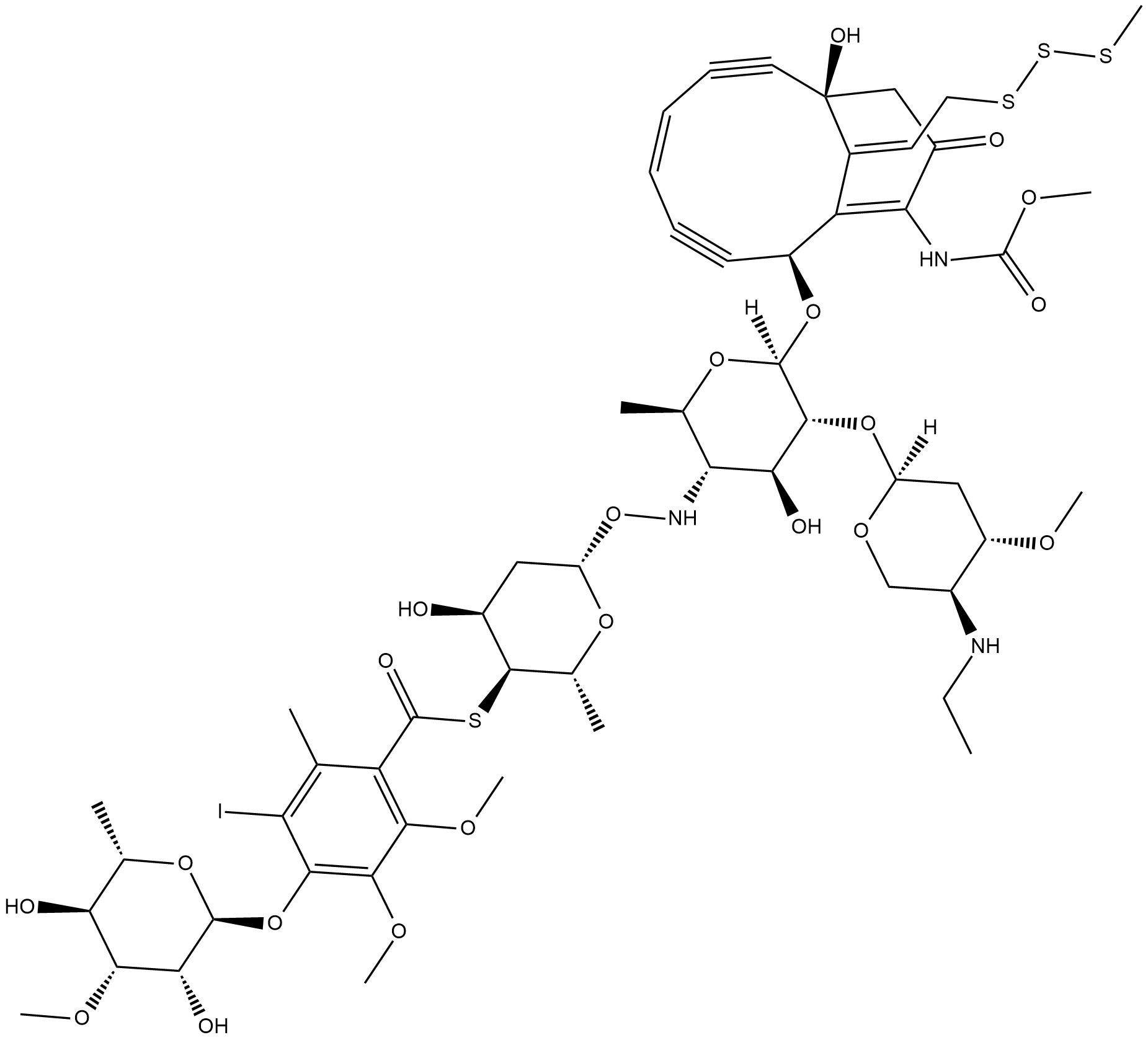
-
GC11207
Carboplatin
Antitumor agent that forms platinum-DNA adducts.
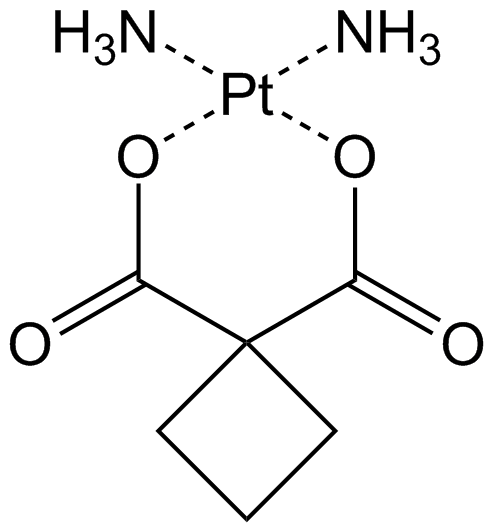
-
GC15793
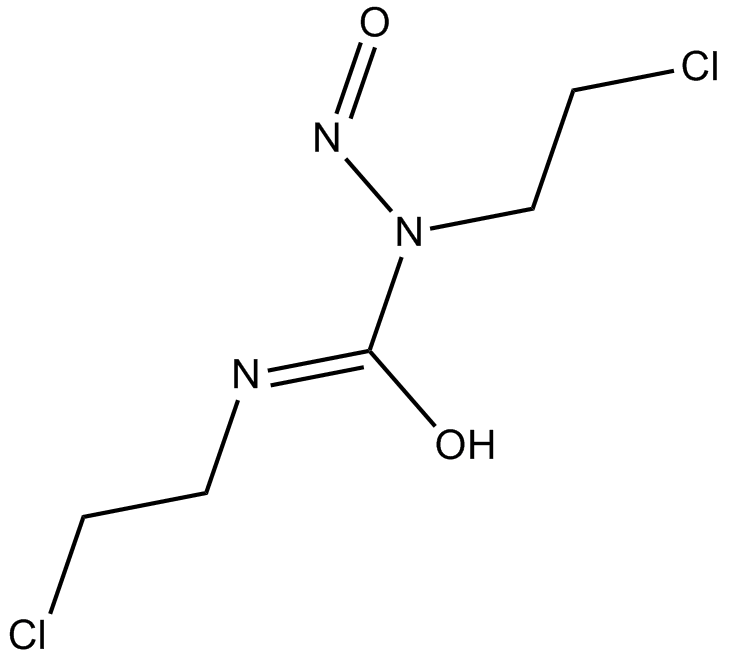
Carmustine
cell-cycle phase nonspecific alkylating antineoplastic agent
-
GC43209
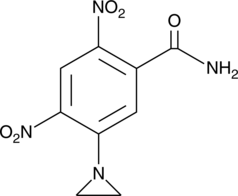
CB-1954
CB-1954 (CB 1954), an antitumor prodrug, is highly selective against the Walker 256 rat tumour line. CB-1954 is enzymatically activated to generate a bifunctional agent, which can form DNA-DNA interstrand cross-links. CB-1954 in rat cells involves the reduction of its 4-nitro group to a 4-hydroxylamine by the enzyme NAD(P)H:quinone oxidoreductase 1 (NQO1).
-
GC12908
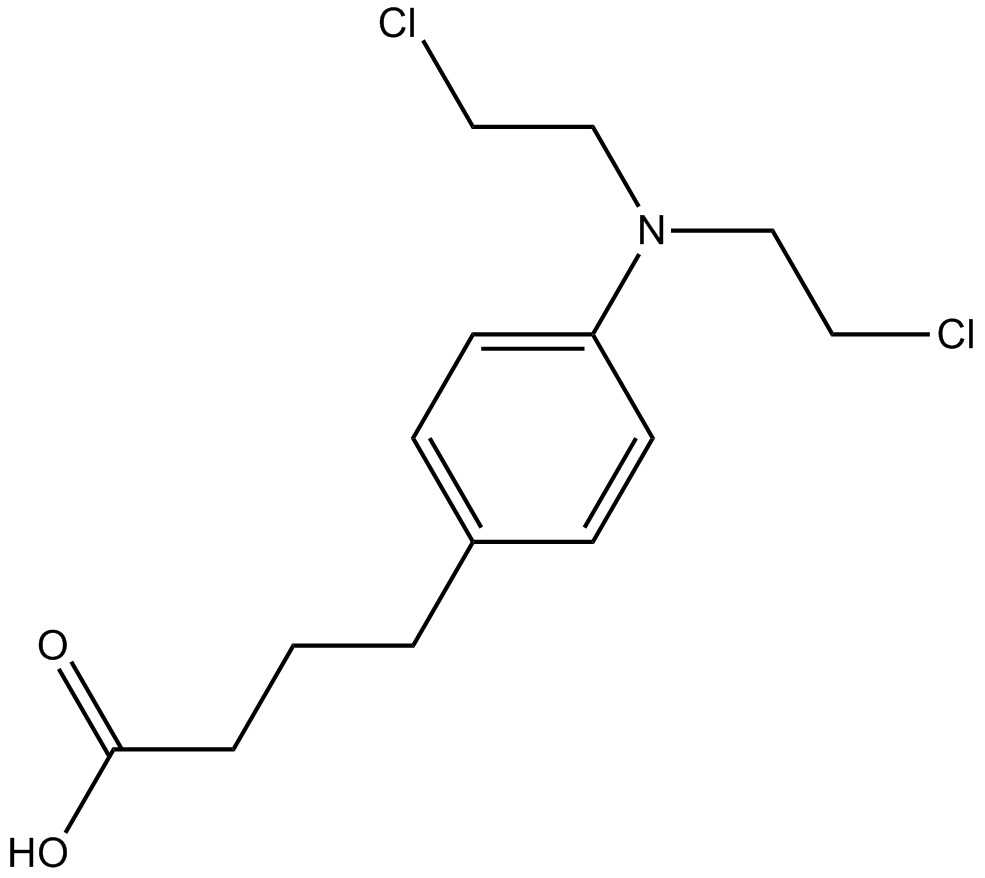
Chlorambucil
nitrogen mustard alkylating agent
-
GC11908
Cisplatin
Cisplatin is one of the best and first metal-based chemotherapeutic drugs, which is used for wide range of solid cancers such as testicular, ovarian, bladder, lung, cervical, head and neck cancer, gastric cancer and some other cancers.

-
GC11145
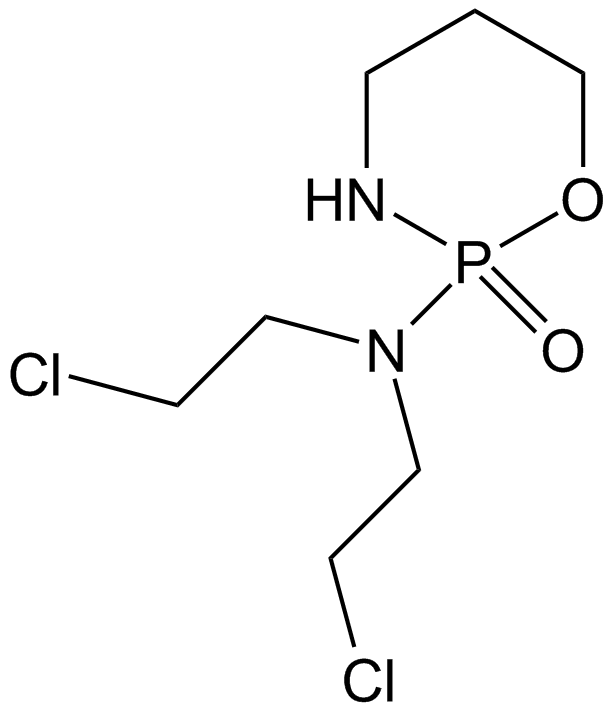
Cyclophosphamide
Cyclophosphamide is a frequently used chemotherapy, often in combination with other chemotherapy types, for the treatment of breast cancer, malignant lymphomas, multiple myeloma, and neuroblastoma
-
GC14077
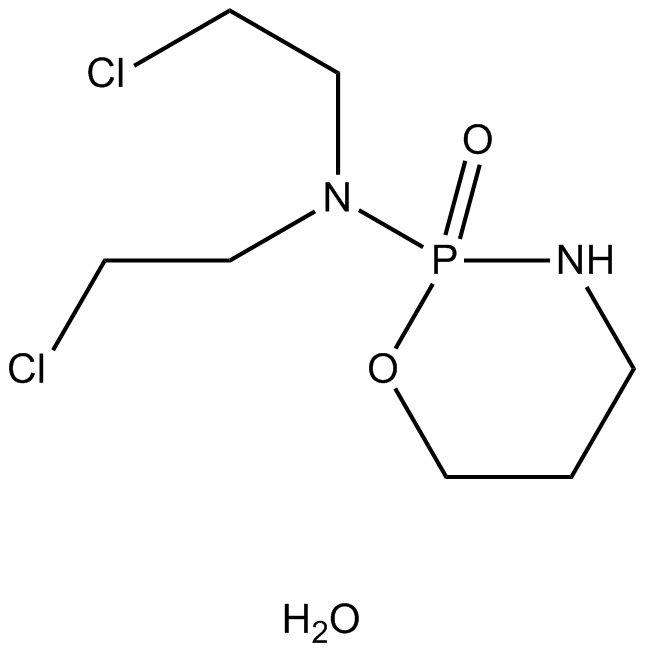
Cyclophosphamide monohydrate
An alkylating agent
-
GC35907
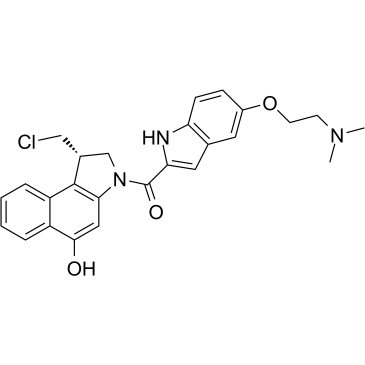
Duocarmycin
Duocarmycin, a DNA minor-groove alkylator, is an antibody drug conjugates (ADCs) toxin. Duocarmycin is based on its characteristic curved indole structure and a spirocyclopropylcyclohexadienone electrophile to act anticancer activity.
-
GC38080
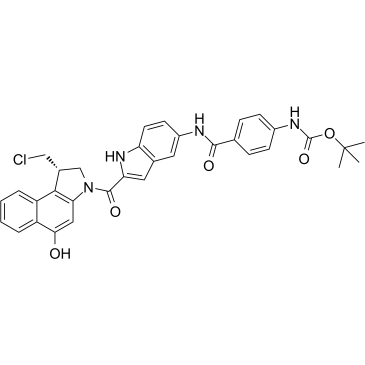
Duocarmycin Analog
Duocarmycin Analog is an analog of Duocarmycin, and used as an DNA alkylator and ADC cytotoxin.
-
GC35909
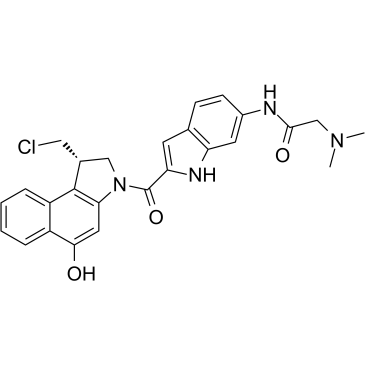
Duocarmycin GA
Duocarmycin GA is an antibody drug conjugates (ADCs) toxin. Duocarmycin is a DNA alkylating agent that binds in the minor groove. Duocarmycin GA can be used against multi-drug resistant cell lines.
-
GC35910
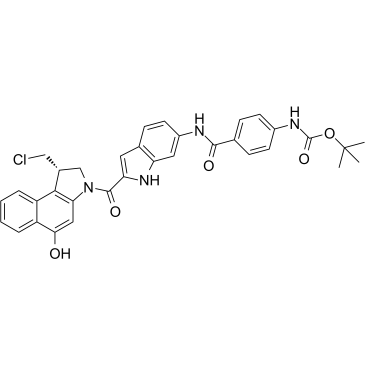
Duocarmycin MA
Duocarmycin MA is an antibody drug conjugates (ADCs) toxin. Duocarmycin is a DNA alkylating agent that binds in the minor groove. Duocarmycin MA can be used against multi-drug resistant cell lines.
-
GC35911
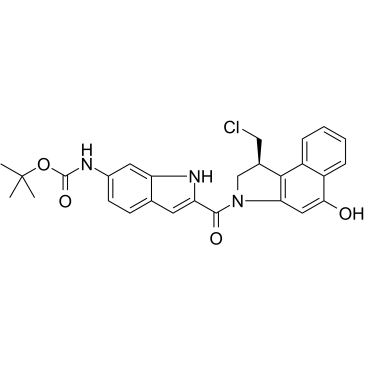
Duocarmycin MB
Duocarmycin MB is an antibody drug conjugates (ADCs) toxin. Duocarmycin is a DNA alkylating agent that binds in the minor groove. Duocarmycin MB can be used against multi-drug resistant cell lines.
-
GC32187
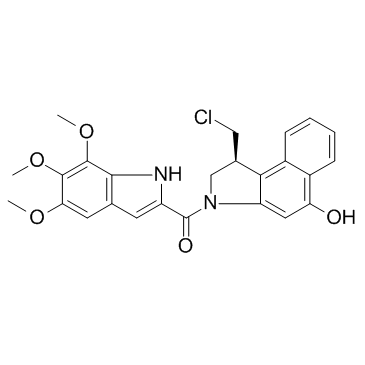
Duocarmycin TM
Duocarmycin TM (CBI-TMI) is a potent antitumor antibiotic. Duocarmycin TM induces a sequence-selective alkylation of duplex DNA.
-
GC16003
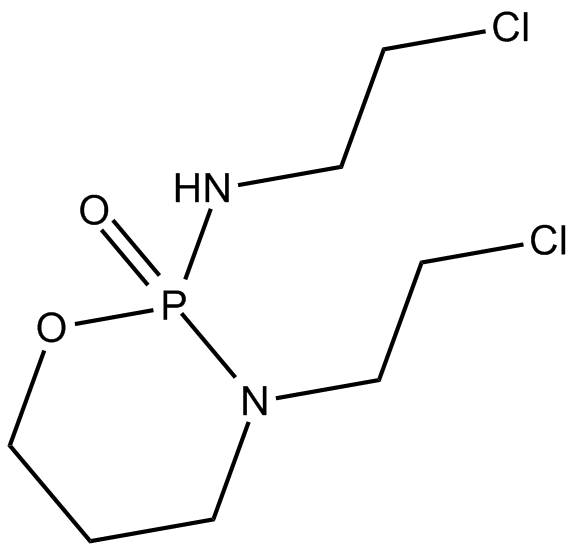
Ifosfamide
Cytostatic agent
-
GC41340
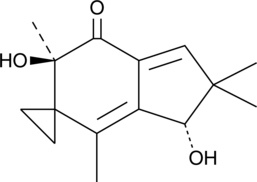
Illudin M
Illudin M is a cytotoxic sesquiterpene from the fungus O.
-
GC41384
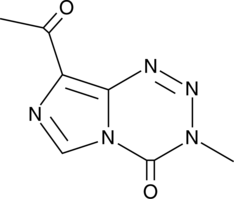
K-TMZ
K-TMZ is a DNA alkylating agent.
-
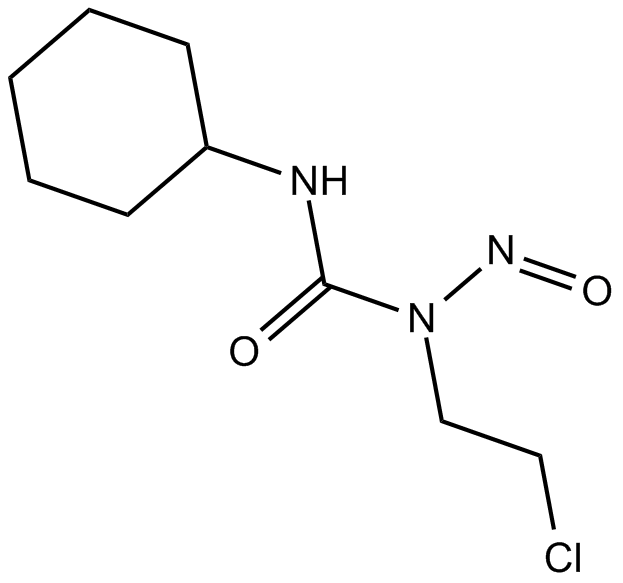
GC17865
Lomustine
Antineoplastic drug
-
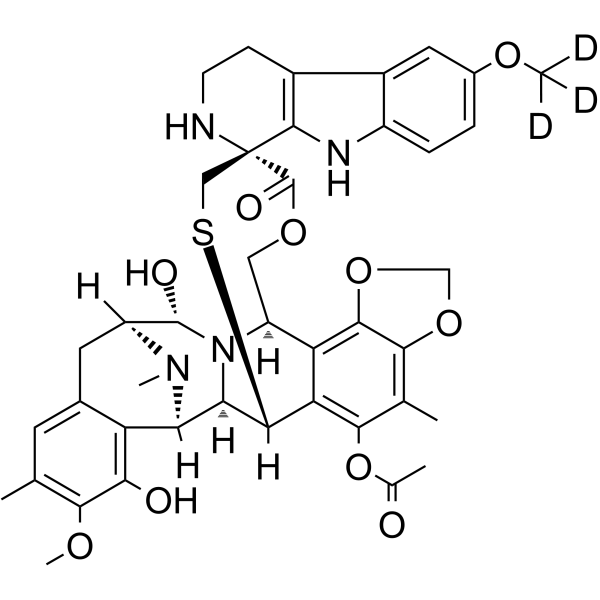
GC63050
Lurbinectedin D3
-
GC12393
Melphalan
DNA alkylating agent
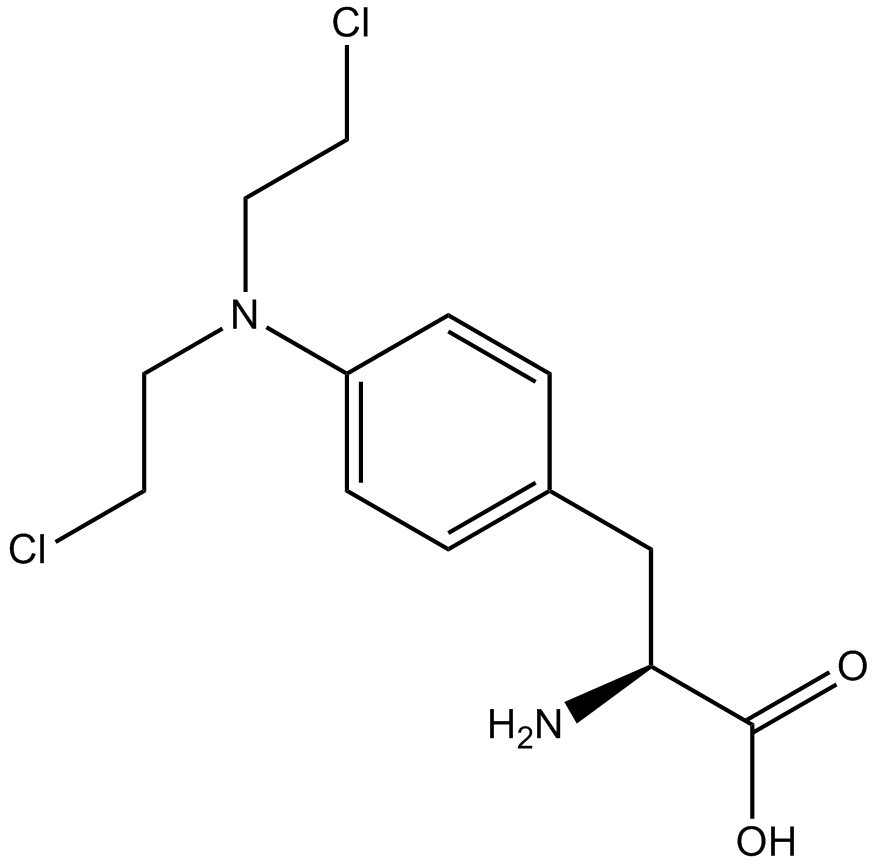
-
GC36598
Methylnitronitrosoguanidine(wetted with ca. 50% Water)
Methylnitronitrosoguanidine(wetted with ca. 50% Water) (MNNG) is an alkylating agent with toxic and mutagenic effects.
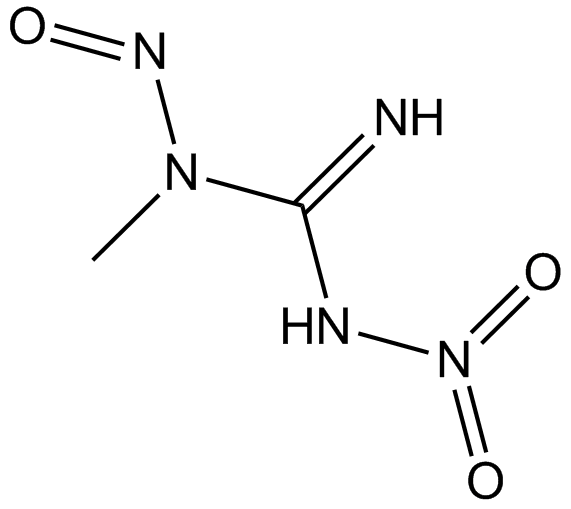
-
GC32886
Miriplatin (SM-11355)
Miriplatin (SM-11355) (SM-11355) is a chemotherapy agent which belongs to the class of alkylating agents.
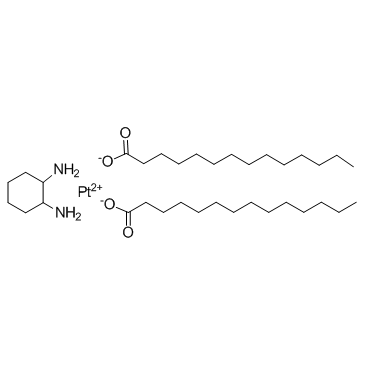
-
GC36615
Miriplatin hydrate
Miriplatin hydrate (SM-11355 hydrate) is a chemotherapy agent which belongs to the class of alkylating agents.
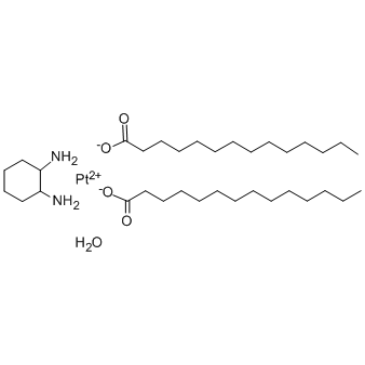
-
GC44243
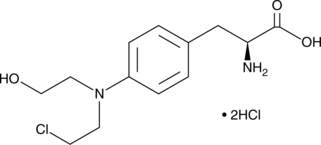
Monohydroxy Melphalan (hydrochloride)
Monohydroxy melphalan is a DNA alkylating agent and a degradation product of melphalan.
-
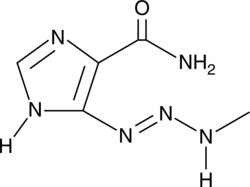
GC44251
MTIC
MTIC is a DNA alkylating agent, an active metabolite of dacarbazine, and an active degradation product of temozolomide.
-
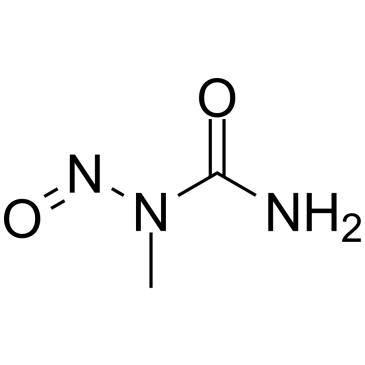
GC39498
N-Nitroso-N-methylurea
N-Nitroso-N-methylurea (NMU;MNU;NMH) is a potent carcinogen, mutagen and teratogenand. N-Nitroso-N-methylurea is a direct-acting alkylating agent that interacts with DNA. N-Nitroso-N-methylurea targets multiple animal organs to cause various cancer and/or degenerative disease. N-Nitroso-N-methylurea is also a precursor in the synthesis of diazomethane.
-
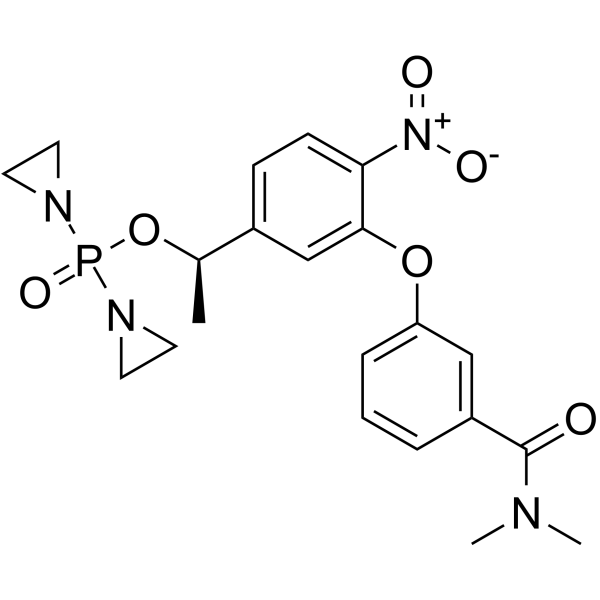
GC62501
OBI-3424
OBI-3424 (TH-3424) is a prodrug that is selectively converted by AKR1C3 (aldo-keto reductase 1C3) to a potent DNA-alkylating agent. OBI-3424 can be used for hepatocellular carcinoma, castrate-resistant prostate cancer, and acute lymphoblastic leukemia (ALL) research.
-
GC17716
Oxaliplatin
Oxaliplatin is a cytotoxic chemotherapy drug used to treat cancer.
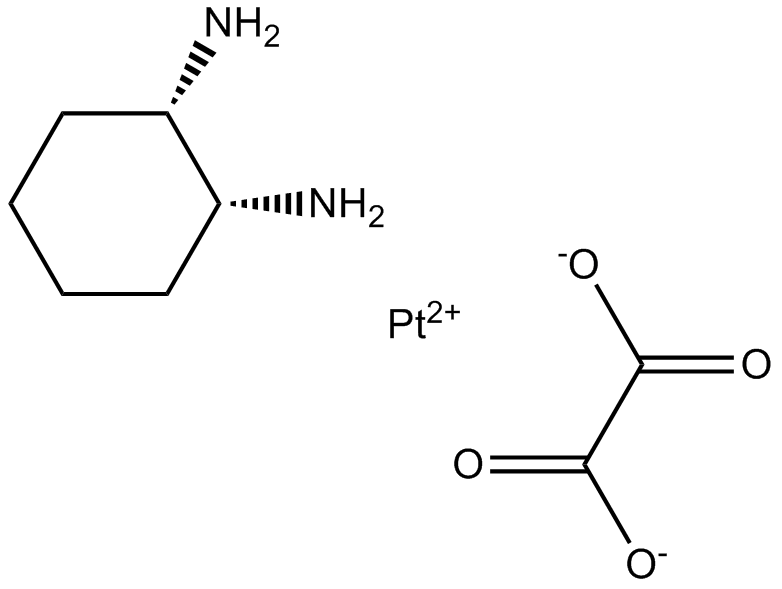
-
GC14762
Palifosfamide
An active metabolite of ifosfamide
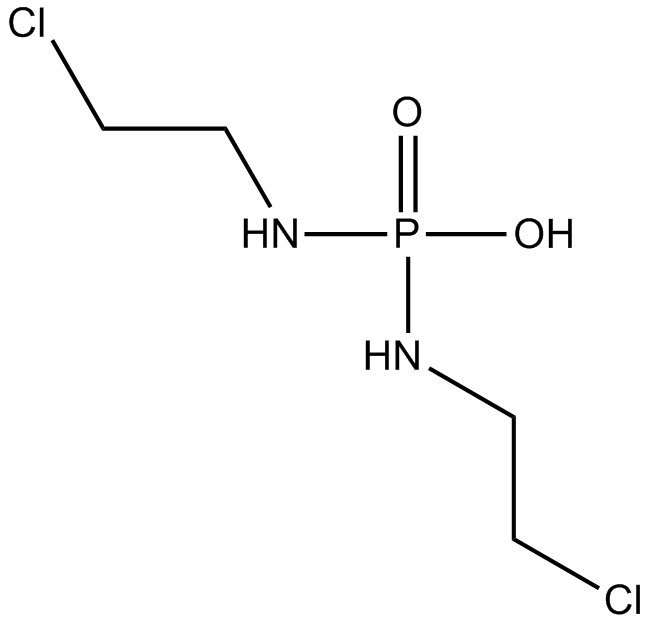
-
GC61182
Phosphoramide mustard (cyclohexanamine)
An alkylating agent and active metabolite of cyclophosphamide
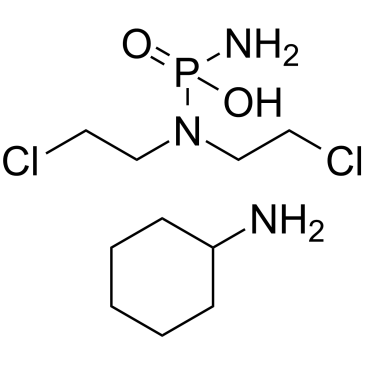
-
GC61188
PIP-199
PIP-199 is a selective inhibitor of RMI (RecQ-mediated genome instability protein) core complex/MM2 interaction, with an IC50 of 36 μM. PIP-199 can be used for the research of sensitizing resistant tumors to DNA crosslinking chemotherapeutics.
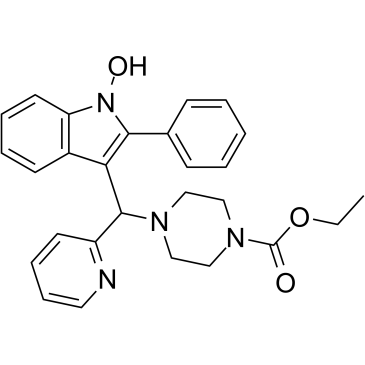
-
GC32946
PK11007
PK11007 is a mild thiol alkylator with anticancer activity. PK11007 stabilizes p53 via selective alkylation of two surface-exposed cysteines without compromising its DNA binding activity. PK11007 induces mutant p53 cancer cell death by increasing reactive oxygen species (ROS) levels.
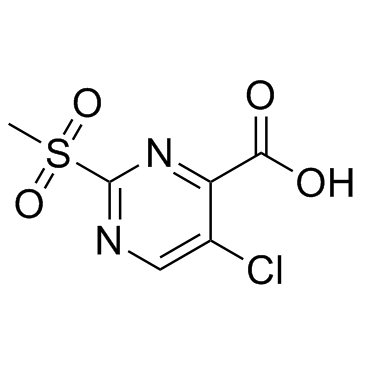
-
GC63154
PR-104
PR-104 is a selective hypoxia-activated DNA cross-linking agent and can be used for the research of multiple tumor xenograft models. PR-104, as a nitrogen mustard pre-prodrug, is converted efficiently to the more lipophilic dinitrobenzamide mustards alcohol PR-104A.
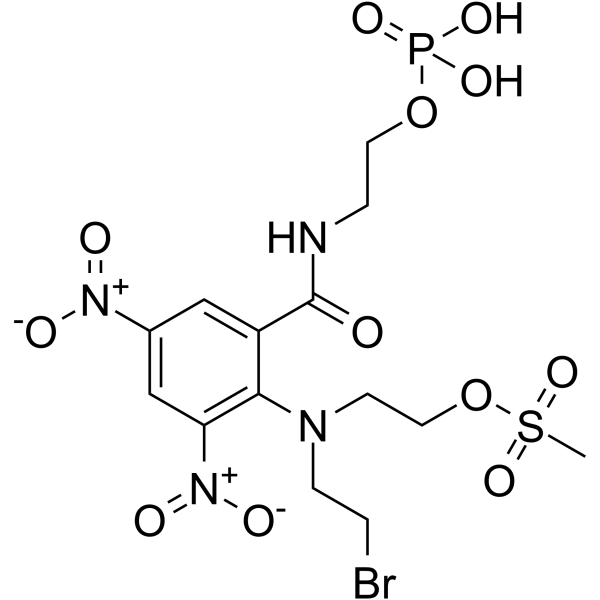
-
GC62490
PR-104A
PR-104A (SN 27858) is the alcohol metabolite of phosphate prodrug PR-104. PR-104A is a hypoxia-selective DNA cross-linking agent/DNA-damaging agent and cytotoxin. Antitumor Activity. PR-104A is metabolized under hypoxia by the 1-electron NADPH:cytochrome P450 oxidoreductase. PR-104A can be used for the research of relapsed/refractory T-lineage acute lymphoblastic leukemia (T-ALL).
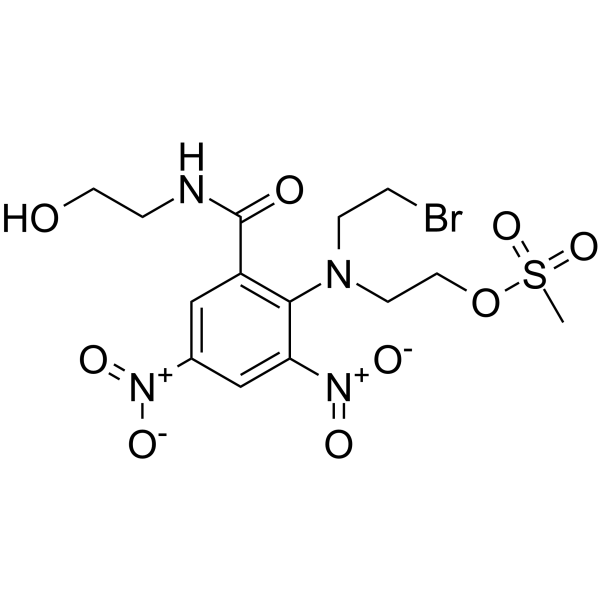
-
GC11793
Procarbazine HCl
Procarbazine HCl is an orally active alkylating agent, with anticancer activity. Procarbazine HCl can be used in Hodgkin's disease research.
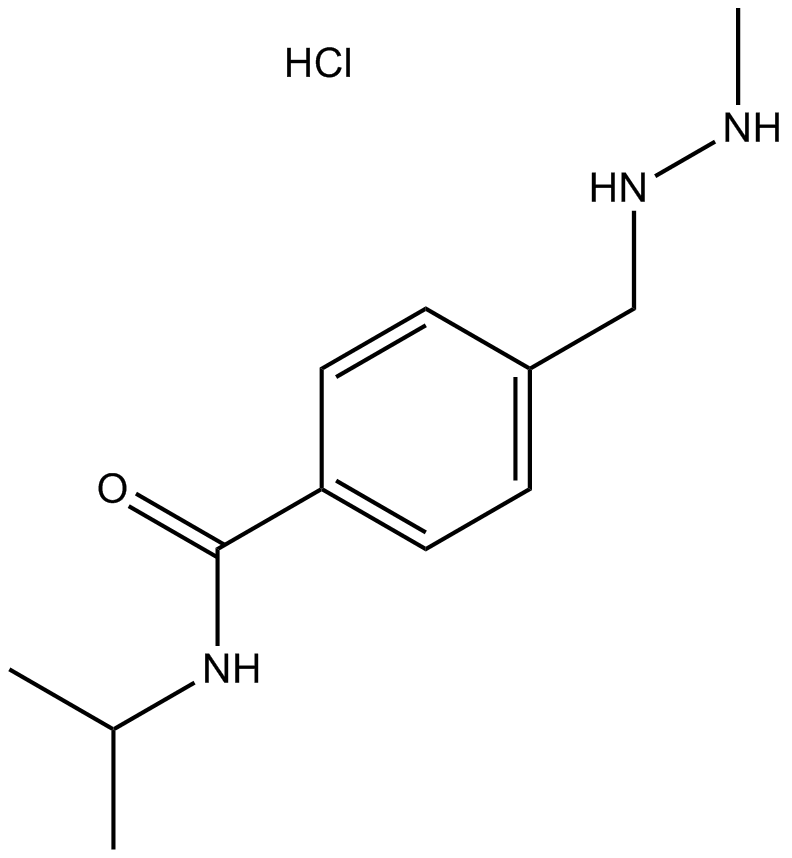
-
GC12793
RITA (NSC 652287)
An inhibitor of the p53-HDM-2 interaction
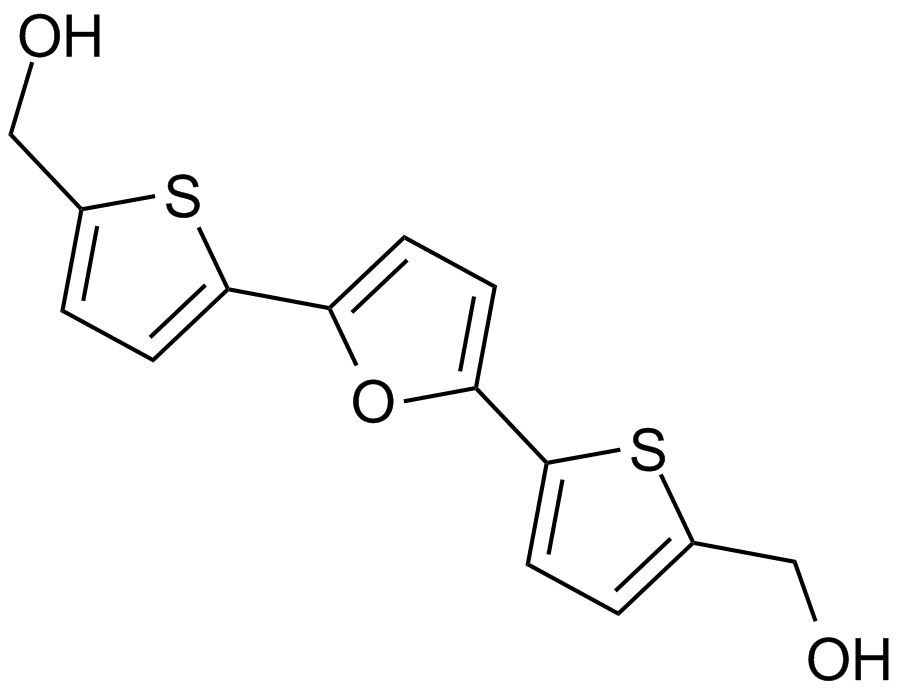
-
GC37593
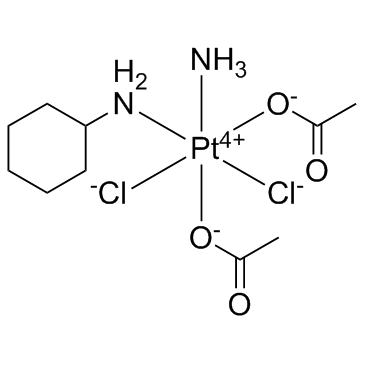
Satraplatin
Satraplatin is an alkylating agent, with potent antitumor effect.
-
GC38349
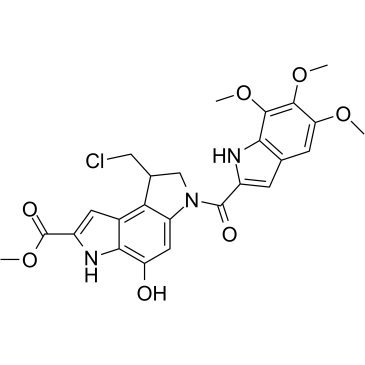
Seco-Duocarmycin SA
Seco-Duocarmycin SA is a DNA alkylator, and is used as an ADC cytotoxin.
-
GC38354
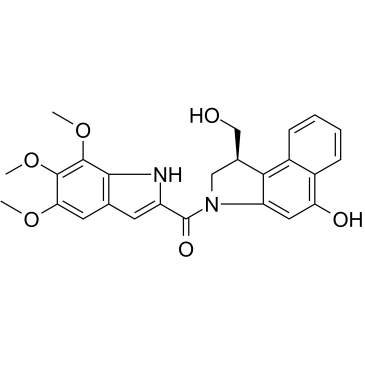
Seco-Duocarmycin TM
Seco-Duocarmycin TM is a DNA alkylator agent belonging to Duocarmycins family that inhibits DNA synthesis. Seco-Duocarmycin TM is a cytotoxic agent, used as the cytotoxic component in antibody-drug conjugates (ADC).
-
GC37625
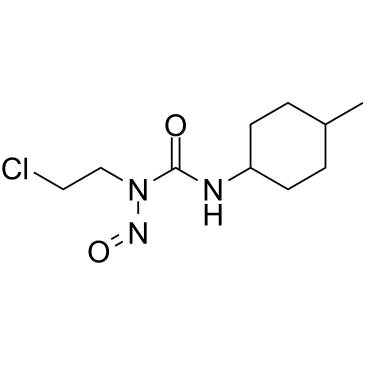
Semustine
Semustine is a DNA alkylator, binds to DNA, and acts as a cancer chemotherapeutic agent.
-
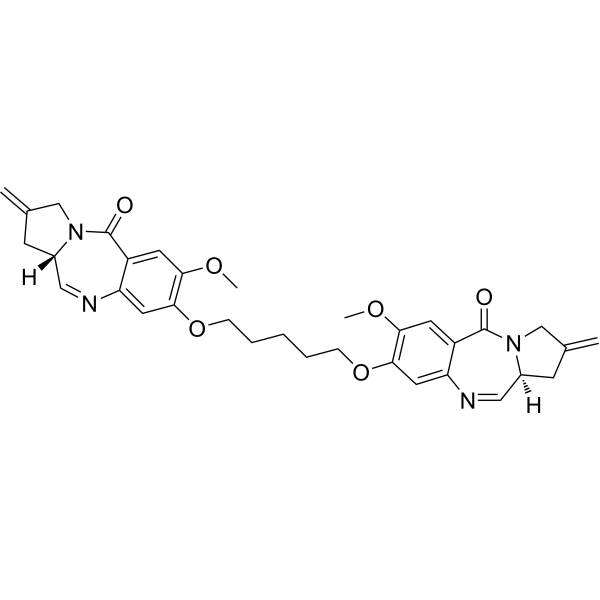
GC69882
SG2057
SG2057 (DRG16) is a PBD dimer containing a pentadiene bond, which selectively binds to sequences in the minor groove of DNA, forming inter- and intra-strand crosslink adducts. SG2057 is an effective anti-tumor agent.
-
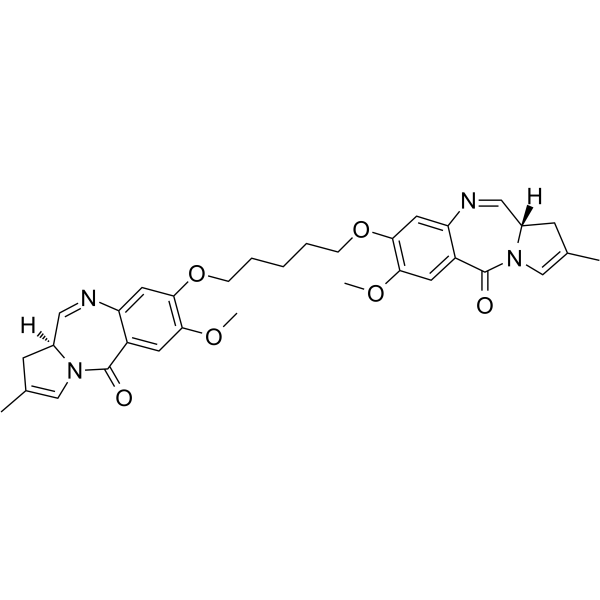
GC62691
SG3199
SG3199 is a cytotoxic DNA minor groove interstrand crosslinking pyrrolobenzodiazepine (PBD) dimer. SG3199 is the released warhead component of the ADC payload Tesirine (SG3249).
-
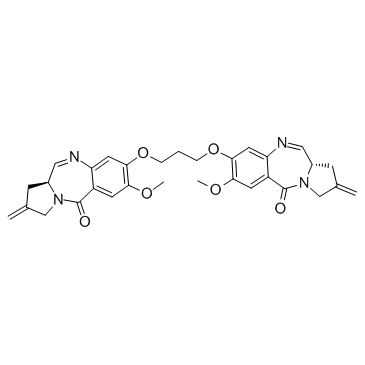
GC34086
SJG-136 (NSC-694501)
SJG-136 (NSC-694501) is a DNA cross-linking agent, with an XL50 of 45 nM for pBR322 DNA. SJG-136 (NSC-694501) has potent antitumor activity.
-
GC17131
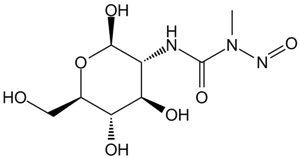
Streptozocin
A diabetogenic agent which targets beta cells
-
GC13667
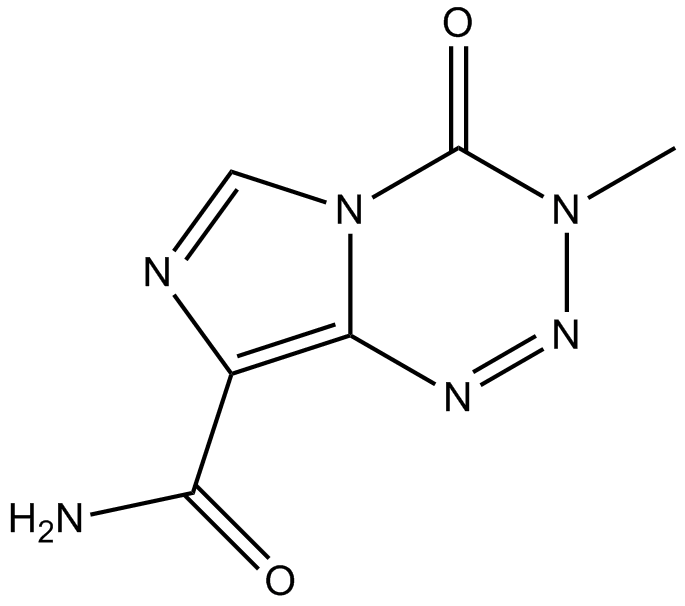
Temozolomide
Temozolomide is an oral activity alkylating agent that induces the formation of O6-methylguanine in DNA
-
GC52076
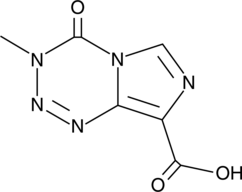
Temozolomide Acid
Temozolomide Acid is a carboxylic acid derivative of Temozolomide. Temozolomide is a DNA alkylating agent, methylating the guanine and adenine bases of DNA, causing breaks in DNA double strand, cell cycle arrest, and eventually cell death. Temozolomide Acid has an activity similar to the parent compound Temozolomide with the same anticancer activity.
-
GC16158
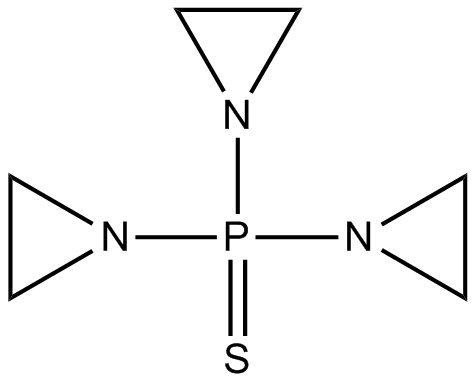
Thio-TEPA
alkylating agent used to treat cancer
-
GC32918
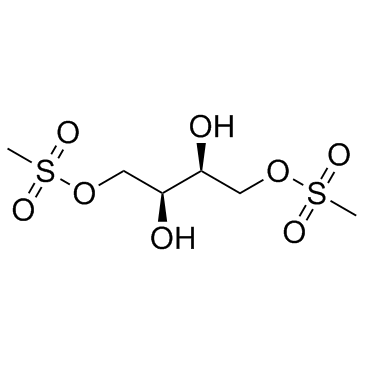
Treosulfan (NSC 39069)
Treosulfan (NSC 39069) (NSC 39069) is a bifunctional alkylating agent with activity in ovarian cancer and other solid tumor types.
-
GC37860
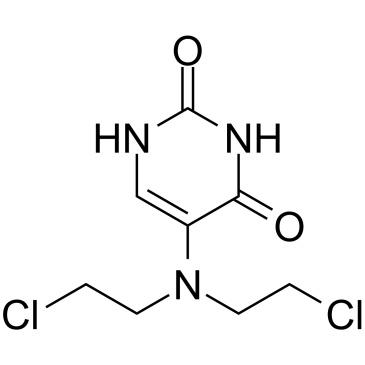
Uramustine
-
GC10339
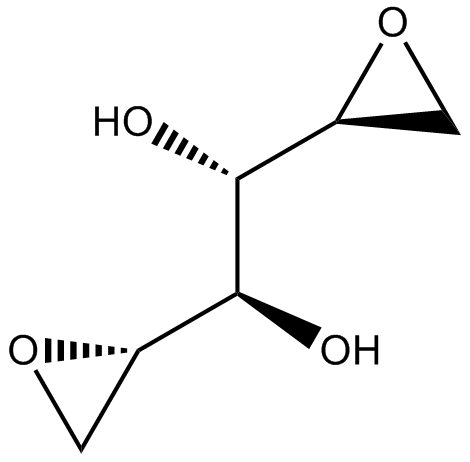
VAL-083