15-keto-17-phenyl trinor Prostaglandin F2α ethyl amide (Synonyms: 15ketoBimatoprost, 17phenyl trinor PGF2α ethyl amide) |
| Catalog No.GC41937 |
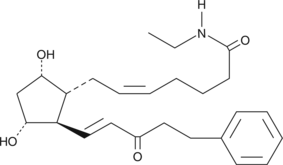
Bimatoprost is the Allergan trade name for 17-phenyl trinor prostaglandin F2α ethyl amide (17-phenyl trinor PGF2α ethyl amide), an F-series PG analog which has been approved for use as an ocular hypotensive drug.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1163135-96-3
Sample solution is provided at 25 µL, 10mM.
Bimatoprost is the Allergan trade name for 17-phenyl trinor prostaglandin F2α ethyl amide (17-phenyl trinor PGF2α ethyl amide), an F-series PG analog which has been approved for use as an ocular hypotensive drug. Oxidation of the C-15 hydroxyl group produces 15-keto-17-phenyl trinor PGF2α ethyl amide. 15-keto-17-phenyl trinor PGF2α ethyl amide is a potential metabolite of 17-phenyl trinor PGF2α ethyl amide when 17-phenyl trinor PGF2α ethyl amide is administered to intact animals. No pharmacological studies on 15-keto-17-phenyl trinor PGF2α ethyl amide have been reported.
Average Rating: 5 (Based on Reviews and 23 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















