Acelarin (NUC-1031) |
| Catalog No.GC34090 |
Acelarin (NUC-1031) (NUC-1031) is a ProTide transformation and enhancement of the widely-used nucleoside analogue, gemcitabine.
Products are for research use only. Not for human use. We do not sell to patients.
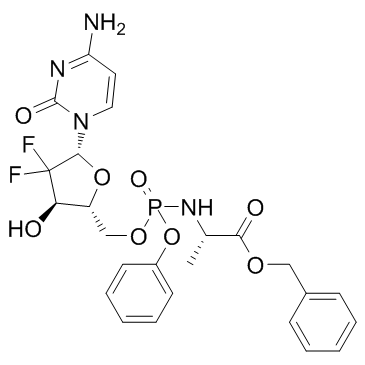
Cas No.: 840506-29-8
Sample solution is provided at 25 µL, 10mM.
Acelarin (NUC-1031) is a ProTide transformation and enhancement of the widely-used nucleoside analogue, gemcitabine.
Gemcitabine is a nucleoside analogue commonly used in cancer therapy but with limited efficacy due to a high susceptibility to cancer cell resistance. The addition of a phosphoramidate motif to the gemcitabine can protect it against many of the key cancer resistance mechanisms. A series of gemcitabine phosphoramidate prodrugs are synthesized and screened for cytostatic activity in a range of different tumor cell lines. Among the synthesized compounds, NUC-1031 is shown to be potent in vitro.
The ProTide demonstrates a significant reduction in tumor size against pancreatic xenograft models compared with the gemcitabine treated group, and less adverse effects on body weight, indicating a better safety profile. Data strongly suggests that the ProTides are not reliant on kinases or nucleoside transporters to exert their activity inside tumor cells and remain stable in the presence of deaminases. The ProTide NUC-1031 is currently advancing through phase I/II clinical studies and has already generated strong pharmacokinetic data that confirm significantly higher intracellular levels of gemcitabine triphosphate, together with promising early efficacy signals and a favorable safety profile. The phosphoramidate chemistry is potentially a great source of new and very effective anticancer agents, bringing a considerable array of advanced treatments specifically designed to overcome cancer resistance mechanisms that will benefit a greater proportion of patients[1].
[1]. Slusarczyk M, et al. Application of ProTide technology to gemcitabine: a successful approach to overcome the key cancer resistance mechanisms leads to a new agent (NUC-1031) in clinical development. J Med Chem. 2014 Feb 27;57(4):1531-42.
Average Rating: 5 (Based on Reviews and 35 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















