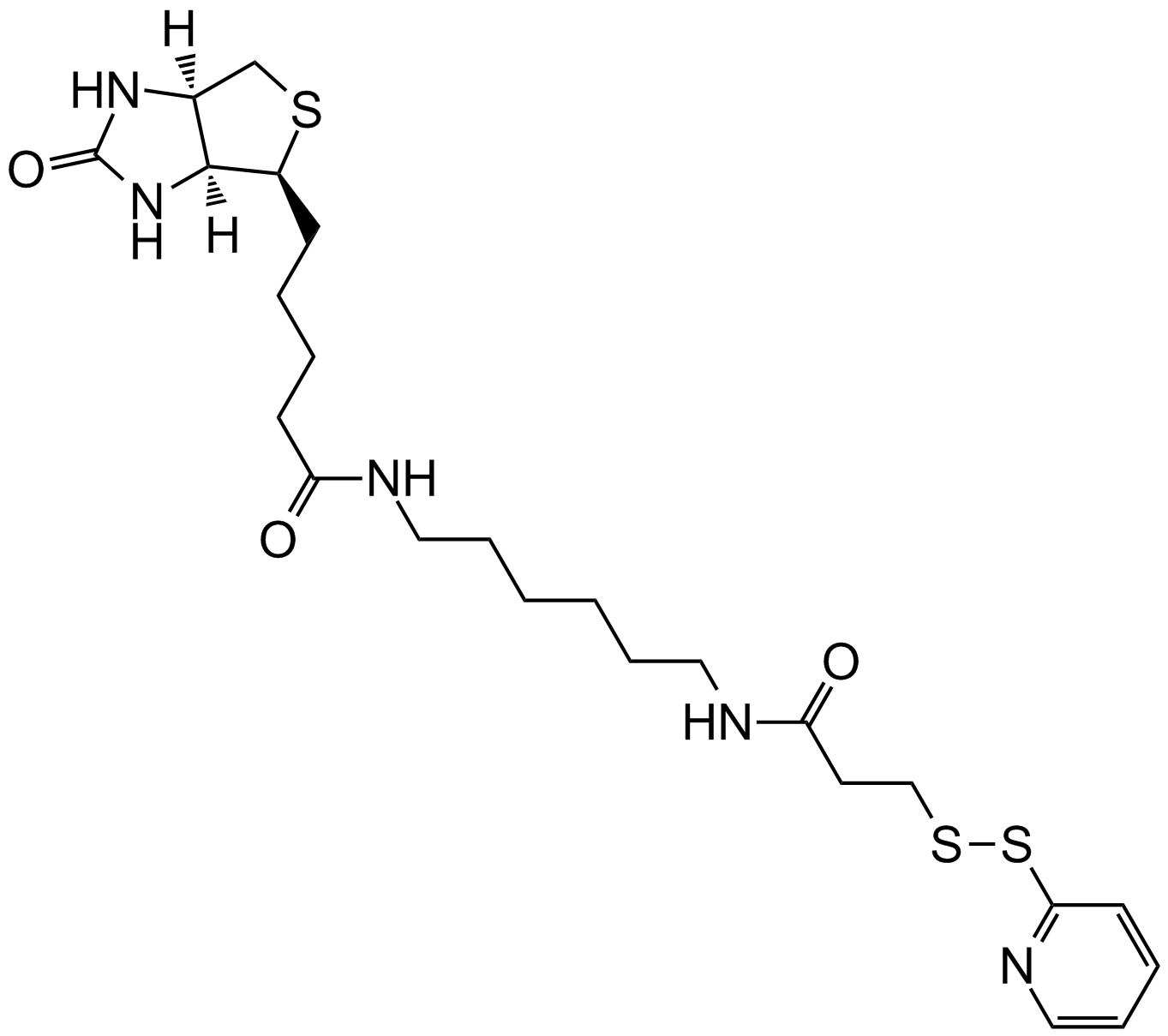
Biotin-HPDP |
| Catalog No.GC11037 |
A sulfhydryl-reactive biotinylation reagent
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 129179-83-5
Sample solution is provided at 25 µL, 10mM.
Biotin-HPDP is a sulfhydryl-reactive biotinylation reagent that forms a reversible disulfide linkage.Biotin-HPDP consists of a bicyclic Biotin ring structure, a 1, 6-diaminohexane attached to the side chain of biotin valonate, and a sulfhydryl reactive group at the end of the side chain[1]. It is used to label protein cysteines and other substrates that contain sulfhydryl groups.
After NEM alkylation, certain (but not all) residual free cysteine residues may react with biotin-HPDP, resulting in the coisolation of proteins containing such cysteine residues[2].
A kinetic study of the modification reactions that generate monothiophosphate disulfide linkages with either 5'-GMPS alone or 5'-GMPS-primed RNA as the substrate revealed that the second-order rate constants increased as the pH was decreased. For example, when the reaction pH was lowered from 8 to 4, the k2 value for the coupling reaction between N-(6-[biotinamido]hexyl)-3'-(2'-pyridyldithio)propionamide (biotin-HPDP) and GMPS increased 67-fold from 1.84 to 123 M(-1) x s(-1) [3].
References:
[1]: Bioconjugate Techniques , 2nd ed. By Greg T.Hermanson ?(Pierce Biotechnology, Thermo Fisher Scientific, Rockford, IL). ?Academic Press ?(an imprint of Elsevier): ?London, Amsterdam, Burlington, San Diego . 2008. ISBN 978-0-12-370501-3.
[2]: Zhou B, Wang Y, Yan Y, Mariscal J, Di Vizio D, Freeman MR, Yang W. Low-Background Acyl-Biotinyl Exchange Largely Eliminates the Coisolation of Non-S-Acylated Proteins and Enables Deep S-Acylproteomic Analysis. Anal Chem. 2019 Aug 6;91(15):9858-9866. doi: 10.1021/acs.analchem.9b01520. Epub 2019 Jul 11. PMID: 31251020; PMCID: PMC7451198.
[3]: Wu CW, Eder PS, Gopalan V, Behrman EJ. Kinetics of coupling reactions that generate monothiophosphate disulfides: implications for modification of RNAs. Bioconjug Chem. 2001 Nov-Dec;12(6):842-4. doi: 10.1021/bc0100612. PMID: 11716671.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




