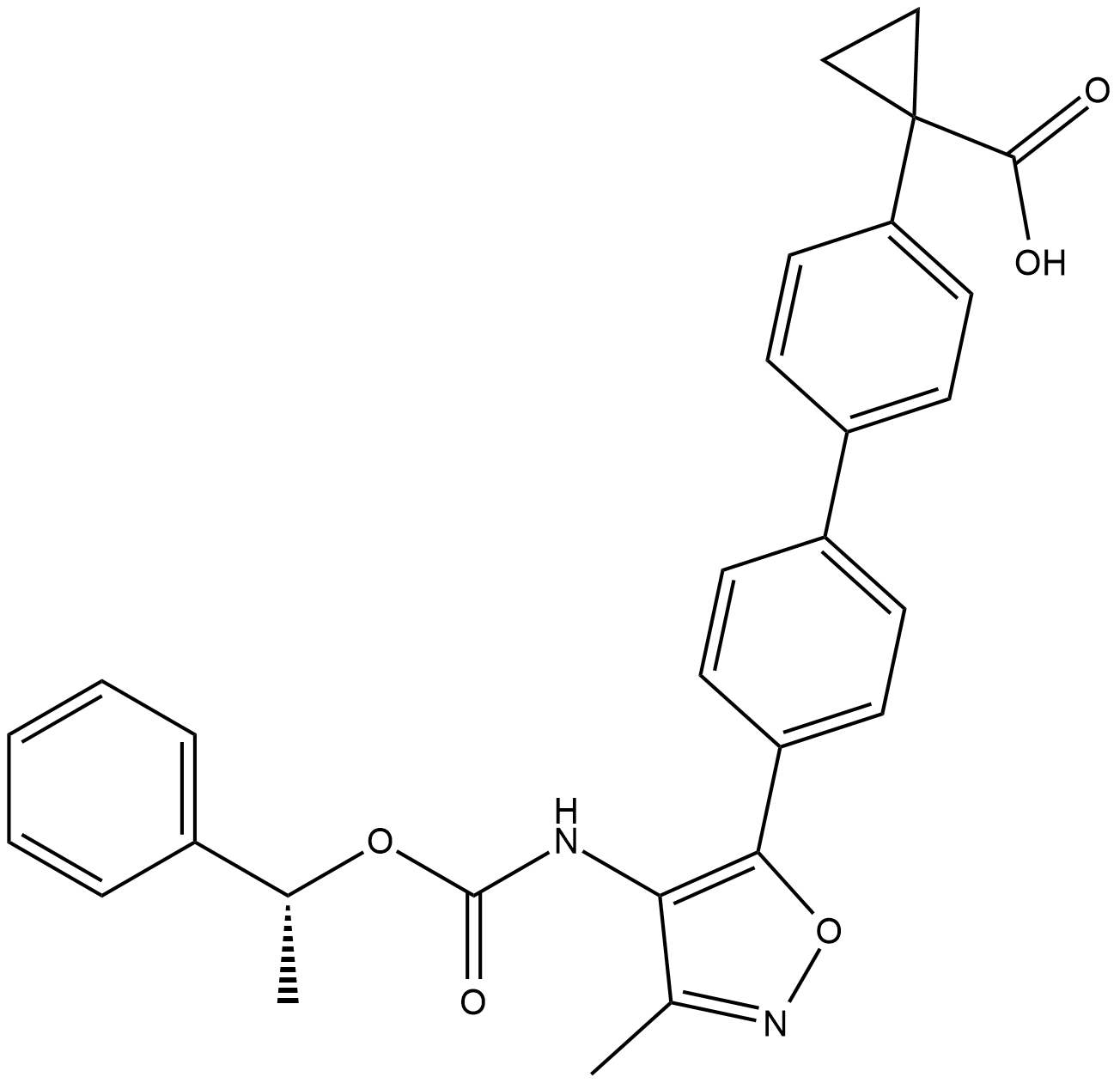
BMS-986278
With a human LPA1Kb of 6.9 nM, the oxycyclohexyl acid BMS-986278 is a strong antagonist of the lysophosphatidic acid receptor 1 (LPA1). We present the structure–activity relationship (SAR) research that began with the therapeutic drug BMS-986020 (1), an LPA1 antagonist, and ended with the discovery of BMS-986278. BMS-986278's comprehensive preclinical pharmacology profiles in vitro and in vivo, along with its pharmacokinetics and metabolic profile, are explained. It was advanced into clinical trials, including an ongoing Phase 2 clinical trial in patients with lung fibrosis, on the basis of its in vivo efficacy in rodent chronic lung fibrosis models and excellent overall ADME (absorption, distribution, metabolism, excretion) properties in multiple preclinical species. The structure of BMS-986278 is show in Figure 1.
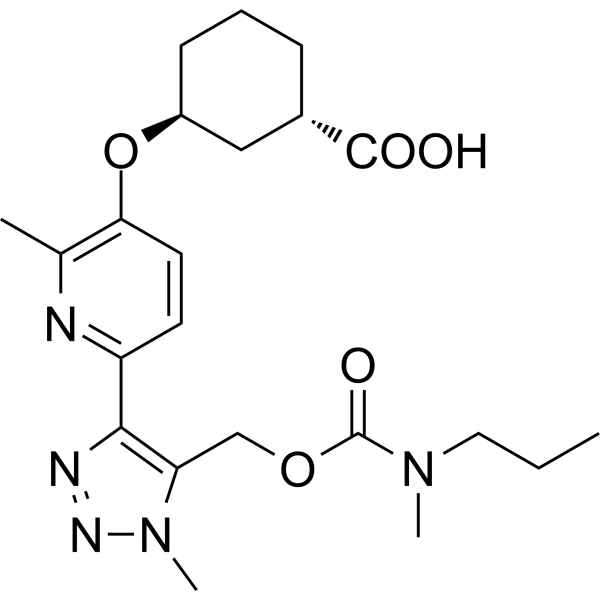
Figure 1: The molecular structure of BMS -986278.
The gradual and deadly illness known as idiopathic pulmonary fibrosis (IPF) is characterized by lung fibrosis that eventually results in lung function loss . Epithelial injury and alterations in the composition of the extracellular matrix (ECM) cause fibroblast activation and migration into the interstitium, collagen buildup, and lung tissue stiffness, which are the hallmarks of interstitial pneumonia . The primary structural proteins in the interstitial matrix, types I and III collagen, undergo significant remodeling in pulmonary fibrosis. The primary component of the basement membrane, which supports the endothelial and epithelial cells lining the blood arteries and airways, is type IV collagen. Type VI collagen is expressed more in IPF-affected lungs than in healthy lungs because it is found at the interface of the interstitial matrix and basement membrane. The evaluation of extracellular matrix turnover yields insights into tissue equilibrium, or the harmony between fibrogenesis and fibrolysis. Neoepitope biomarkers, which measure newly formed epitopes that may be generated by removal of collagen propeptides (reflecting protein formation) or by specific protease-mediated cleavage of mature proteins (reflecting protein degradation), can be used to evaluate ECM remodeling in fibrotic conditions such as IPF. According to earlier research, LPA1 antagonistic effects may be directly antifibrotic, especially in cases of lung fibrosis. In a phase 2 clinical trial, BMS-986020, an oral bioavailable LPA1 antagonist of the first generation, showed proof-of-mechanism in patients with IPF. Generally, BMS-986020 decreased the reduction in forced expiratory volume (FVC) when compared to a placebo for 26 weeks. Significant differences were observed after 600 mg BID was administered. The majority of patients tolerated BMS-986020 well, however three patients experienced hepatobiliary symptoms that resulted in cholecystectomy, which led to the termination of the trial program. Subsequent in vivo and in vitro tests established that BMS-986020 alone was responsible for the reported hepatobiliary damage, with no connection to LPA1 antagonistic activity. BMS-986278, a second-generation LPA1 antagonist, is presently being developed in phase 2 trials for patients with progressive fibrotic interstitial lung disease and IPF. It has not demonstrated hepatobiliary toxicity in nonclinical evaluations in phase 1 studies. Using macromolecular crowding to increase collagen formation and collagen crosslinking in cultured fibroblasts, Scar-in-a-Jar is an in vitro three-dimensional (3D) model of fibrogenesis (Fig. 2). This work employed an extended system to examine BMS-986020's impact on ECM-neoepitope biomarkers in greater detail.
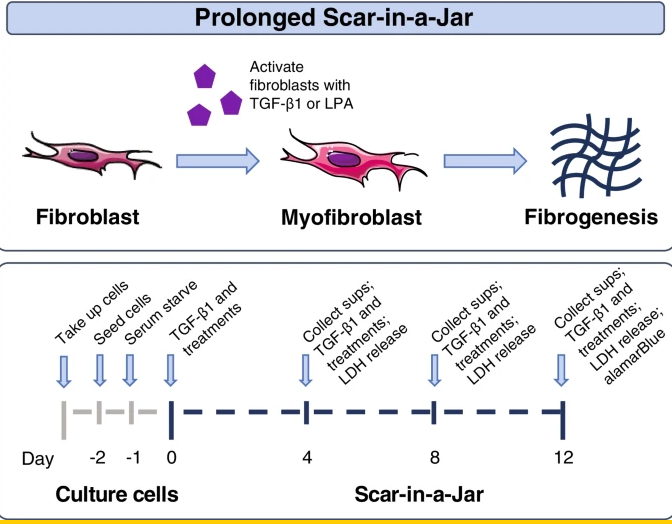
Figure 2: Scar-in-a-Jar experimental system. LDH lactate dehydrogenase; LPA lysophosphatidic acid; Sups supernatants; TGF-β1 transforming growth factor-beta 1
In IPF patients, QLF and FVC were linked with ECM-neoepitope biomarker levels. According to Figures 3A, C, and D, there was a substantial baseline correlation between whole lung QLF and PRO-C4 (r = 0.311, P < 0.001) and C6M (r = 0.397, P < 0.001). All lung locations showed (Figure 3) a general tendency of positive connection between baseline ECM-neoepitope biomarkers and baseline fibrosis, even though not all correlations were statistically significant after multiple testing correction.
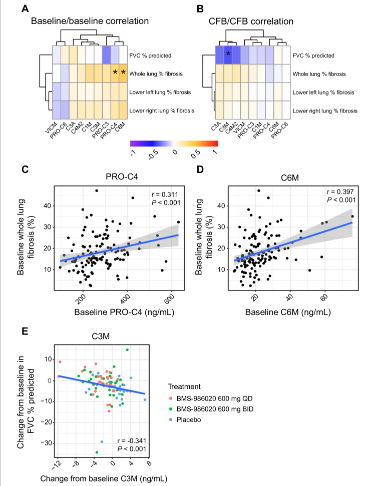
Figure 3: Heatmaps and scatterplots and linear regression of ECM-neoepitope biomarker levels and pulmonary measures. A Heatmap of pairwise Spearman correlation of baseline ECM-neoepitope biomarker levels with baseline FVC and fibrosis measurements.
Baseline ECM-neoepitope levels mainly overlapped when stratified by positive versus negative change in fibrosis and FVC, suggesting a limited prognostic power on disease development over 26 weeks as evaluated by FVC in this investigation.
The effect of BMS-986020 on serum ECM-neoepitope biomarkers over time was examined after the association between FVC and lung fibrosis was established and the degree to which ECM-neoepitope biomarkers correlated with pulmonary function and fibrosis was described. In a linear mixed model analysis, BMS-986020 therapy significantly decreased serum levels of all ECM-neoepitope indicators (Figure 4), except for PRO-C3 and PRO-C6 when compared with placebo at Week 26.
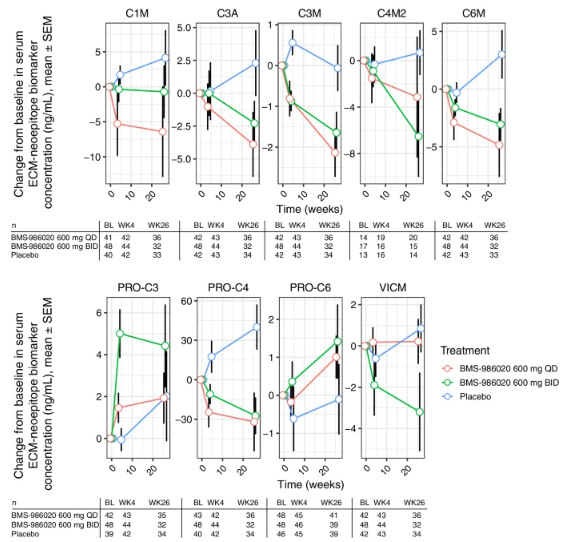
Figure 4: ECM-neoepitope biomarker CFB measurements in patients with IPF from the phase 2 trial NCT01766817. Patient numbers for each ECM-neoepitope biomarker stratified by treatment group and time point are indicated.
It is possible that the unexpected elevations in PRO-C3 and PRO-C6 were related to the hepatobiliary toxicity reported in the group treated with BMS-986020, given the established association between these two proteins and liver dysfunction. Week 26 CFB in PRO-C3 was substantially linked with changes in aspartate aminotransferase (AST) and direct bilirubin levels in the BMS-986020 treatment groups. Direct bilirubin level was also linked with week 26 CFB in PRO-C6. Conversely, there was no positive correlation seen between changes in liver enzyme levels and collagen degradation indicators.
Using the extended Scar-in-a-Jar in vitro fibrogenesis paradigm, the effects of LPA and LPA1 antagonism on ECM-neoepitope biomarkers were directly evaluated. The Scar-in-a-Jar investigations assessed biomarkers of collagen production (PRO-C1, PRO-C3, and PRO-C6), α-SMA, and FBN-C. However, because collagen-degrading proteases were absent from the system, it was not possible to investigate biomarkers of ECM breakdown. In the culture supernatant, LPA raised the levels of -SMA, FBN-C, PRO-C1, PRO-C3, and PRO-C6 in comparison to no stimulation (Figure 5). Compared to TGF-β1, LPA caused reduced levels of α-SMA, PRO-C1, and PRO-C3 (all days), and greater levels of FBN-C (Days 8 and 12) and PRO-C6 (all days). The Kruskal-Wallis test, in conjunction with the P value false discovery rate correction, indicated significant differences in the means for each biomarker, stimulation, and timepoint trio in 27 out of 30 combinations.
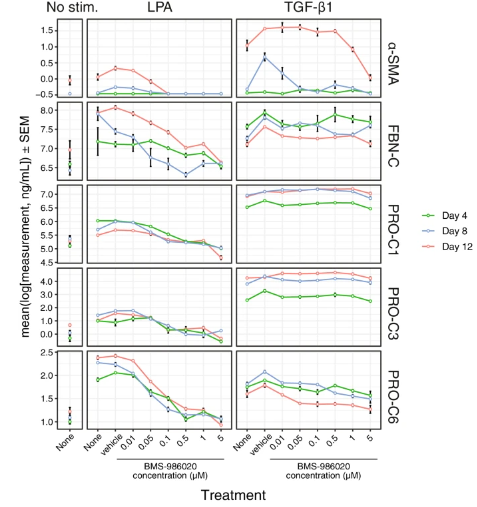
Figure 5: Effects of BMS-986020 on LPA- or TGF-β1–stimulated fibrogenesis in the Scar-in-a-Jar in vitro model. Shown are ECM-neoepitope biomarkers over time for untreated, vehicle-, and BMS-986020–treated fibroblasts.
On Day 12, the LPA-stimulated (P < 0.01) and unstimulated (P < 0.05) cells showed BMS-986020–mediated reductions in metabolic activity as assessed by alamarBlue; however, these effects were at higher doses and were less pronounced than the effects on biomarker production. The BMS-986020-treated unstimulated cells also showed noticeably slower increases in ECM-neoepitope biomarker levels over time. Other tests revealed that BMS-986020 was non-cytotoxic, with no effect on LDH release at up to 10 μM.
The information presented here extends the reach of pharmaceutical LPA1 antagonistic effects to collagen turnover in IPF patients, hence supporting their antifibrotic properties. Moreover, better results for FVC and QLF are linked to the reductions in C3M and C6M levels brought on by BMS-986020, respectively.












Comments