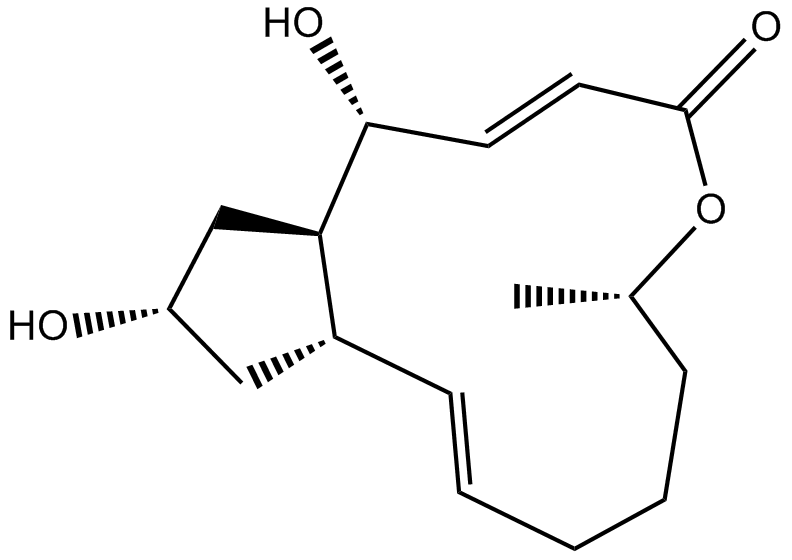
Brefeldin A
Cytotoxin Produced by diverse kinds of fungi e.g Paecilomyces sp. And Aspergillus clavatus isolated from Taxus mairei and Torreya grandis from Southeast china, 12,15-Dihydroxy-7-methyl-8-oxabicyclo[11.3.0]hexadeca-2,10-dien-9-one also called as Brefeldin A (BFA) is a lactone antibiotic and a specific inhibitor of protein trafficking. Brefeldin A blocks the transport of secreted and membrane proteins from endoplasmic reticulum to Golgi apparatus. Brefeldin A is also an autophagy and mitophagy inhibitor. It was reported to inhibit protein and nucleic acid synthesis. It’s physical appearance is White to off-white crystalline powder. It's chemical formula is C16H24O4 and Molar mass is 280.36 g/mol.
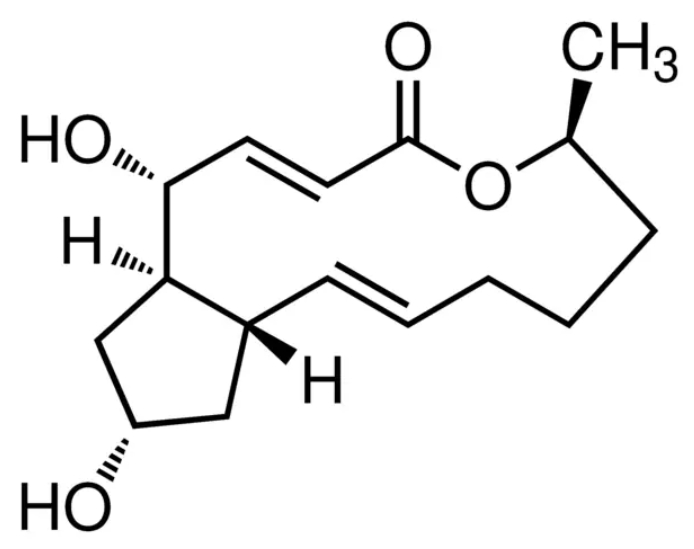
Figure 1: Demonstrates the structure of Brefeldin A.
Brefeldin A possesses a diverse spectrum of antifungal,antiviral, antimitotic and antitumor activity. It is now well established that the target of BFA in mammalian cells is a subset of Sec7-type GTP exchange factors (GEFs) that catalyze the activation of a small GTPase called Arf1p.BFA-sensitive GEFs are localized to the Golgi apparatus of mammalian and yeast cells.Molecular targets for BFA also exist in plant cells. The Arabidopsis gene GNOM encodes an Arf GEF that falls into the subgroup of BFA-sensitive Sec7-like Proteins .BFA has similar primary effects in plants as in animals.
If we talk about the mechanism of action of Brefeldin A it causes Blockade of intracellular protein trafficking and don’t effect its synthesis. Later studies indicate that BFA inhibits GDP/GTP exchange reaction on adenosine ribosylation factors (ARFs), and as such prevents association of coat protein (β -COP) and blocks transport vesicle formation.
Let’s talk about the mechanism how does Brefeldin A inhibit ARF1 exchange factors? The characterization of the mutations present in GEA1 alleles resistant to Brefeldin A shows that the altered positions are clustered on a region of Sec7 domain overlapping with, or very close to, the ARF binding domain . So , we can say that Brefeldin A completes with ARF for binding to sac7 guanine nucleotide exchange domain .If that was true it was real example of small molecule blocking a protein , protein interaction. So mechanism of Brefeldin A is of great importance in drug discovery and basic biology.
But recent studies show that Brefeldin A stabilizes an ARF:Sec7 domain complex rather than preventing its formation. It showed that Brefeldin A is an uncompetitive inhibitor. It binds to the transient complex formed between ARF-GDP and the Sec7 domain, leading to an abortive ARF-GDP:brefeldin A:Sec7 domain complex.brefeldin A makes endogenous ARF1-GDP that traps the exchange factor and blocks activation of other ARF1 molecules by this particular exchange factor.
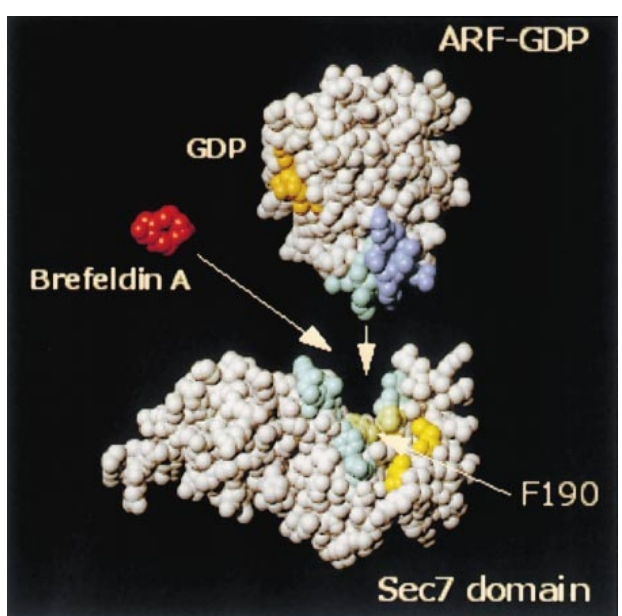
Figure 2: Mechanism of Brefeldin A inhibition.
Another prominent characteristic of BFA action is fragmentation of the Golgi and TGN and induction of their merge with the ER and lysosome/endosome. The dissociation of coat protein from Golgi membrane is an early event in the induction of retrograde Golgi-to-ER membrane flow, and is thought to be a prerequisite for the induction of Golgi fragmentation.
We postulate that in plant as in mammalian cells, BFA leads to a breakdown of the physical separation of ER and Golgi compartments by allowing membrane fusion to occur in the absence of prior vesicle formation and not by a stimulation of the normal retrograde vesicular transport pathway.BFA-induced fusion is a pathological event that occurs only after prior dissociation of COPI from the Golgi and culminates in the physical continuity and mixing of the ER
and the Golgi apparatus. Brefeldin A inhibits vesicle formation and transport between the endoplasmic reticulum and the Golgi apparatus which ultimately results in collapse of the Golgi apparatus into the endoplasmic reticulum via membrane fusion.Let us now examine the effect of BFA on membrane trafficking .
Untreated cells exported from the ER is mediated by COPII-coated vesicles . Fusion of these anterograde vesicles depends on anterograde v-SNAREs on the vesicles and t-SNAREs on the cis Golgi . Anterograde transport continues on the trans side of the Golgi with Catherin-coated vesicles (crossed blue lines). Retrograde transport within the Golgi and from the Golgi to the ER depends on COPI coats and retrograde v- and t-SNAREs (black triangles and lines). In BFA-treated cells (right), all vesiculation at the Golgi stops due to the inhibition of Arf1. As a result, retrograde v-SNAREs remain exposed on the Golgi and the cisternae fuse directly with the ER. Cisternal maturation continues in the presence of BFA so that early Golgi compartments assume a more trans-like morphology. At the same time, the later cisternae and the TGN are lost to the cytoplasm and, eventually, to the BFA compartment. COPII vesicle formation at the ER is initially not inhibited by BFA, but anterograde vesicles may no longer be able to fuse with the maturing cis cisterna, thus effectively blocking ER-to-Golgi transport. As shown in the figure 3.
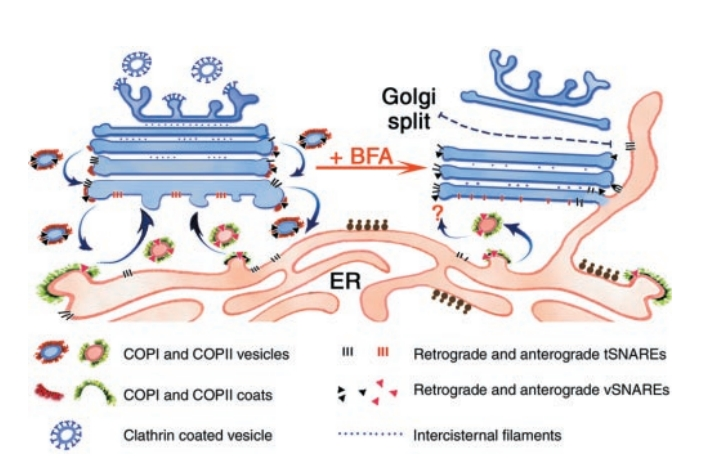
Figure 3: Effect of Brefeldin A on membrane trafficking between ER and the Golgi.
Let us know about the Effects of Brefeldin A on Intracellular Processing of Haptoglobin.Haptoglobin was initially synthesized as prohaptoglobin with M, = 45,000, which was immediately cleaved into the alpha and beta subunits. Beta Subunit with M, = 33,000 containing high mannose type Oligosaccharide chains was further processed to a M, = 36,000 with the complex type oligosaccharide chains, which was secreted into the medium (Fig. 4A). Upon treatment of the cells with Brefeldin A (1 pg/ml), the precursor with M, = 45,000 observed just after pulse-label disappeared with the same time course as in the control cells, while the M, = 36,000 form of the beta subunit did not appear until 2 h of chase (Fig. 4B). The intracellularly accumulating beta subunit with M, =33,000 was found to have the high mannose type oligosaccharide chains, since it was sensitive to Endo H. The beta subunit, however, was finally secreted into the medium after being converted to the M, = 36,000 form with the complex type sugar chains. These results indicate that the drug has no effect on the proteolytic conversion of prohaptoglobin into the subunits, but exerts the same inhibitory effect on the processing in glycosylation of the beta-subunit as observed for alpha protease inhibitor.
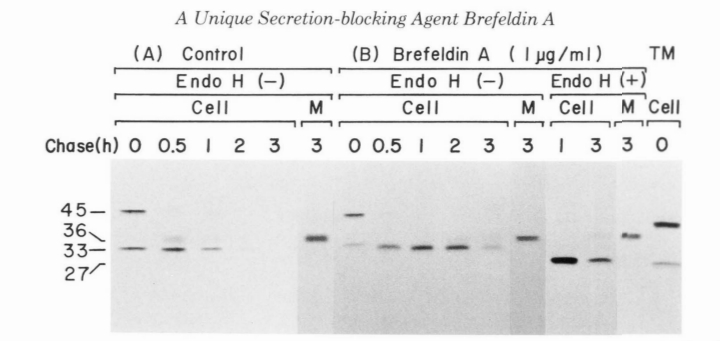
Figure 4: shows the effect of Brefeldin A on the intracellular processing of Haptoglobin
The antiviral activity of Brefeldin A is remarkable in vitro, especially with the Agar-diffusion plaque-inhibition method. It is characteristic of Brefeldin A that it forms large plaque-free protected zones without cytotoxicity. In this assay system Brefeldin A is effective against herpes simplex virus strain HF (HSV) as well as NDV so that both DNA and RNA viruses are included in the antiviral spectrum of Brefeldin A. For example BFA inhibits DENV at an early phase in the viral replication cycle after viral entry. BFA is an antiviral candidates against DENV-2 in culture cell.
Brefeldin A is an antibiotic with antifungal and cytotoxic activity in vitro as well as antitumor activity in Vivo against some experimental tumors . Anti -viral activity of Brefeldin A was determined by Plaque-inhibition agar-diffusion method.Inhibition method. Brefeldin A forms large plaque-free protected zones without cytotoxicity (Table 1). In this assay System Brefeldin A is effective against herpes Simplex virus strain HF (HSV) as well as NDV so that both DNA and RNA viruses are included in its antiviral spectrum.
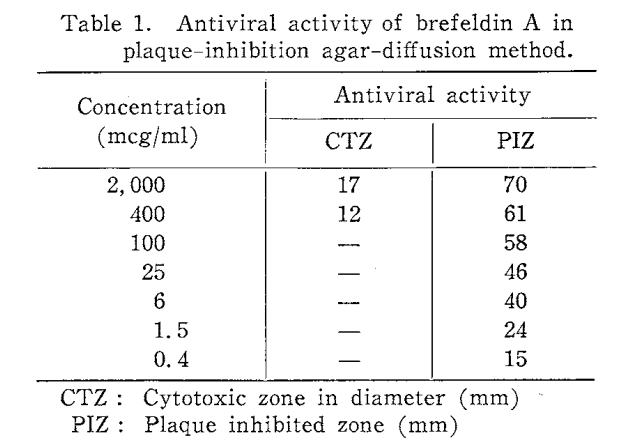
The Paper-disc used absorbed only 0.05ml of Solvent per disc so 0.1mg of Brefeldin A gave a 70mm diameter zone free form of plaque formation by NDV.To conclude ,Brefeldin A (BFA) has been shown to interfere with number of cellular functions, including alteration of the function of the Golgi and trans-Golgi network, disruption of the traffic between endosomes and lysosomes, and inhibition of protein secretion and synthesis because of its impairment of vesicular transport. Brefeldin A is not considered to be harmful from direct skin or eye exposure other than transient irritation. It may cause irritation of the respiratory system if inhaled.













Comments