Brefeldin A (Synonyms: Ascotoxin, BFA, Cyanein, Decumbin, Nectrolide, NSC 56310, NSC 89671, NSC 107456, NSC 244390, Synergisidin) |
| Catalog No.GC17683 |
Brefeldin A (BFA) is a fungal macrocyclic lactone and a potent, reversible inhibitor of intracellular vesicle formation and protein trafficking between the endoplasmic reticulum (ER) and the Golgi apparatus.
Products are for research use only. Not for human use. We do not sell to patients.
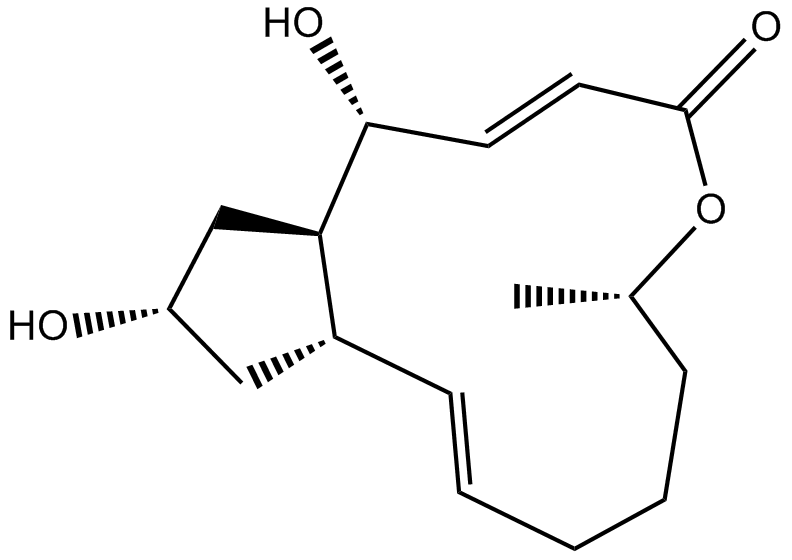
Cas No.: 20350-15-6
Sample solution is provided at 25 µL, 10mM.
Brefeldin A (BFA) is a fungal macrocyclic lactone and a potent, reversible inhibitor of intracellular vesicle formation and protein trafficking between the endoplasmic reticulum (ER) and the Golgi apparatus[1][2].Brefeldin A is an ATPase inhibitor with IC50 value of 0.2 µM[9].Brefeldin A and its analogs are promising inhibitors in drug development due to a number of key features such as apoptosis?inducing properties as well as antitumor, antifungal, and antiviral effects [3,6].Brefeldin A is a CRISPR/Cas9 activator. Brefeldin A inhibits HSV-1 and has anti-cancer activity[4].
Perturbation of ER-Golgi trafficking by brefeldin A (BFA) treatment attenuated nucleotide-binding oligomerization domain-like receptor family, pyrin-domain-containing 3 (NLRP3) inflammasome activation in mouse bone marrow-derived macrophages (BMDMs) [5].ADP-ribosylation of BARS is mediated by formation of a conjugate between Brefeldin A and ADPR. BARS shows BAC binding when incubated with the medium from the brefeldin A-treated CD38+ HeLa cells[3].Brefeldin A induces anchorage-independent cell death in MDA-MB-231 breast cancer cells with an EC50 of 0.016 µg/mL, inhibits the formation of MDA-MB-231 colonies in 3D and 2D cultures and inhibits the migration and MMP 9 activity of MDA-MB-231[2].
In tumor-bearing mice, M-brefeldin A can prolong blood circulation, improve tumor accumulation ability, and show effective inhibition of tumor growth, M-brefeldin A 10 mg/kg group showed effective antitumor effect and significantly delayed tumor progression, while M-brefeldin A 5 mg/kg mice did not show significant inhibitory effect[7]. Mice were treated with the Golgi blocker Brefeldin A. Since most cytokines are processed and secreted via the classical secretion pathway through the Golgi, brefeldin A blocks cytokine secretion, leading to their accumulation within immune cells, which are eventually detected by flow cytometry. Thus, treatment of mice with brefeldin A allows in situ assessment of cytokine production without the use of reporter mice [8].
References:
[1]: Orci L, Tagaya M, et,al. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183-95. doi: 10.1016/0092-8674(91)90273-2. PMID: 2004424.
[2]: Tseng CN, Hong YR, et,al. Brefeldin A reduces anchorage-independent survival, cancer stem cell potential and migration of MDA-MB-231 human breast cancer cells. Molecules. 2014 Oct 29;19(11):17464-77. doi: 10.3390/molecules191117464. PMID: 25356567; PMCID: PMC6271931.
[3]: Wang J, Fang Y, et,al. Erythroleukemia cells acquire an alternative mitophagy capability. Sci Rep. 2016 Apr 19;6:24641. doi: 10.1038/srep24641. PMID: 27091640; PMCID: PMC4835698.
[4]: Yu C, Liu Y, et,al. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell. 2015 Feb 5;16(2):142-7. doi: 10.1016/j.stem.2015.01.003. PMID: 25658371; PMCID: PMC4461869.
[5]: Hong S, Hwang I, et,al. Brefeldin A-sensitive ER-Golgi vesicle trafficking contributes to NLRP3-dependent caspase-1 activation. FASEB J. 2019 Mar;33(3):4547-4558. doi: 10.1096/fj.201801585R. Epub 2018 Dec 28. PMID: 30592629.
[6]: Paek SM. Recent Synthesis and Discovery of Brefeldin A Analogs. Mar Drugs. 2018 Apr 18;16(4):133. doi: 10.3390/md16040133. PMID: 29670019; PMCID: PMC5923420.
[7]: Zhang JM, Jiang YY, et,al. Brefeldin A delivery nanomicelles in hepatocellular carcinoma therapy: Characterization, cytotoxic evaluation in vitro, and antitumor efficiency in vivo. Pharmacol Res. 2021 Oct;172:105800. doi: 10.1016/j.phrs.2021.105800. Epub 2021 Aug 4. PMID: 34363949.
[8]: Kovacs SB, Oh C, et,al. Evaluating cytokine production by flow cytometry using brefeldin A in mice. STAR Protoc. 2020 Dec 30;2(1):100244. doi: 10.1016/j.xpro.2020.100244. PMID: 33458706; PMCID: PMC7797915.
[9]: Wierzbicki PM, Kogut-Wierzbicka M, et,al. Protein and siRNA delivery by transportan and transportan 10 into colorectal cancer cell lines. Folia Histochem Cytobiol. 2014;52(4):270-80. doi: 10.5603/FHC.a2014.0035. Epub 2014 Dec 16. PMID: 25511292.
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




