GIP (Human)
GIP is the short form of either Gastric Inhibitory Polypeptide or Glucose-dependent Insulinotropic Polypeptide. The primary structure of Glucose-dependent Insulinotropic Polypeptide is composed of a linear sequence of 42 residual amino acids; that is determined by the Edman Degradation Method and well documented in the scientific literature. The 3-letter notation of the primary structure of Glucose-dependent Insulinotropic Polypeptide is given in Figure 1.

Figure 1 The 3-letter notation of the Primary Structure of Glucose-dependent Insulinotropic Polypeptide
For the secondary structure of Glucose-dependent Insulinotropic Polypeptide GIP, strong region with the high probability of formation of the alpha helices as a secondary structure have been identified which are present from 10 to 29 residual amino acid. This prediction of the secondary structure is based on the experimental results of the Gascuel and Golmard Basic Statistical Method via the computer-assisted approach, and are indicated as grey in the illustration given in Figure 2. On top of that, the Glucose-dependent Insulinotropic Polypeptide GIP is prone to be attacked by a number of enzymes namely DP4, V8, cyanogen bromide CNBr, enterokinase EK, and Trypsin, etc. normally present in the human body, as binding sites for them exist on the structure of Glucose-dependent Insulinotropic Polypeptide GIP and are indicated by the arrows in the illustration given in Figure 2.
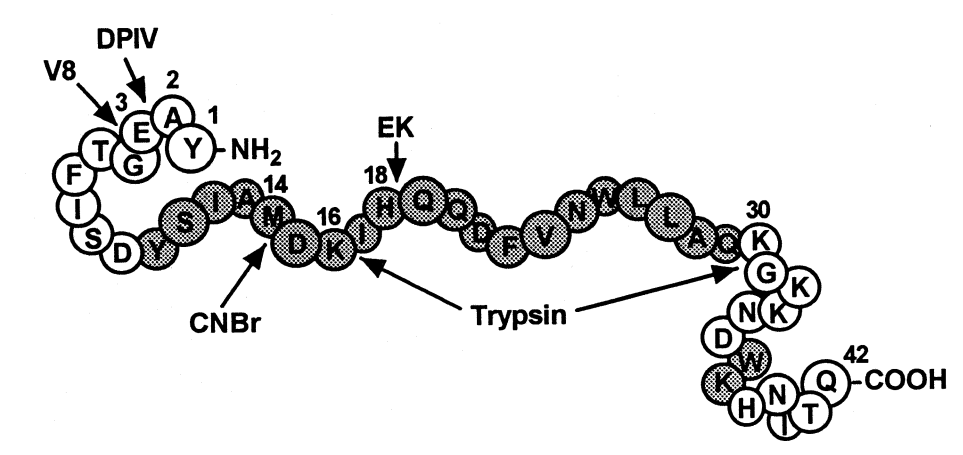
Figure 2 The Secondary Structure of Glucose-dependent Insulinotropic Polypeptide GIP: Strong region for the formation of alpha helices is indicated from 10 to 29 residual amino acid, in grey bubbles of the 1 letter notation of Amino Acids. In addition, the sites for different enzymes, i.e., DP4, V8, cyanogen bromide CNBr, , enterokinase EK, and Trypsin, are also indicated with the help of arrows which makes the overall structure of the Glucose-dependent Insulinotropic Polypeptide GIP very susceptible for the enzymatic degradation via the bodily enzymes.
Under the normal physiological conditions, upon the oral food ingestion, which is primarily a glucose intake considering on the nutrient level, Glucose-dependent Insulinotropic Polypeptide GIP is produced and released by the K-cells in the mucosa epithelial layer of small intestines majorly in the duodenum and jejunum regions of the digestive system in the human body; upon its release it stimulates the production and release of insulin. This phenomenon is called as Incretin Effect, which is also known as Insulinotropic effect. As the K-cells are the enteroendocrine cells which are of open type cell; direct physical contact with the glucose molecules can initiate the production and release of Glucose-dependent Insulinotropic Polypeptide GIP. This underlying physiology accounts for its name as Glucose-dependent Insulinotropic Polypeptide. In the K-cells, Glucose-dependent Insulinotropic Polypeptide GIP is formed after the PC-1/3 processing of pro-GIP, which is a peptide having the length of 153 residual amino acids. The newly formed GIPs in fully intact form, are temporarily stored in the granules before their release. Once released, these GIPs are instantaneously degraded via the enzymatic activity of dipeptidyl peptidase 4, shortened as DPP-4; therefore, either GIP 1-42 which is the normal/ undegraded form or GIP 3-42 which is the degraded form sometimes regarded as inactive form, is present in the blood circulation. While, on the other hand, the dysregulated production/ release of Glucose-dependent Insulinotropic Polypeptide GIP is associated with pathological condition; elevated levels of Glucose-dependent Insulinotropic Polypeptide GIP are a major characteristic of Type 2 diabetes and decreased levels of Glucose-dependent Insulinotropic Polypeptide GIP is a major characteristic of insulin resistance. Therefore, production and release of Glucose-dependent Insulinotropic Polypeptide GIP is a clinically significant physiological phenomenon.
Another physiological activity that is mediated by Gastric Inhibitory Polypeptide GIP is the inhibition of both, the production and release of the gastric acid. This phenomenon is called enterogastrone effect. This underlying physiology accounts for its name as Gastric Inhibitory Polypeptide GIP. Being its proteinaceous structure and involvement in either stimulatory or inhibitory roles in the body, it is regarded as hormone; On top of that, it is biosynthesized in the human body as a part of metabolism, it is more appropriately regarded as a metabolic hormone.
Only the fully intact Glucose-dependent Insulinotropic Polypeptide GIP interacts with, binds to and activates its receptor namely GIP receptor as shown in Figure 3 and 4, which is a G protein coupled receptor that belongs to Class B of the family of heptahelical GPCRs. Interestingly, not all the amino acid residues of the Glucose-dependent Insulinotropic Polypeptide GIP are interacting with the GIP receptor; the amino acid residues 1-15 interacts with the transmembrane domain of the GIP receptor via the formation of a dense network of hydrogen bonds with three cores that is illustrated in Figure 5 and 6 and the amino acid residues 16-30 interacts with the extracellular domain, while the amino acid residues 31-42 do not interact with either type of domains, i.e., transmembrane domain or extracellular domain. In the overall interaction between the Glucose-dependent Insulinotropic Polypeptide GIP and GIP receptor, the N terminal of the Glucose-dependent Insulinotropic Polypeptide GIP, which is positively charged, plays the most significant role; thus, this N terminal is critical for the subsequent activation of GIP receptor and demonstration both physiological effects, i.e., enterogastrone effect and insulinotropic effect. On the other hand, there is no significant role of the C terminal in the interaction of activation of the GIP receptor.
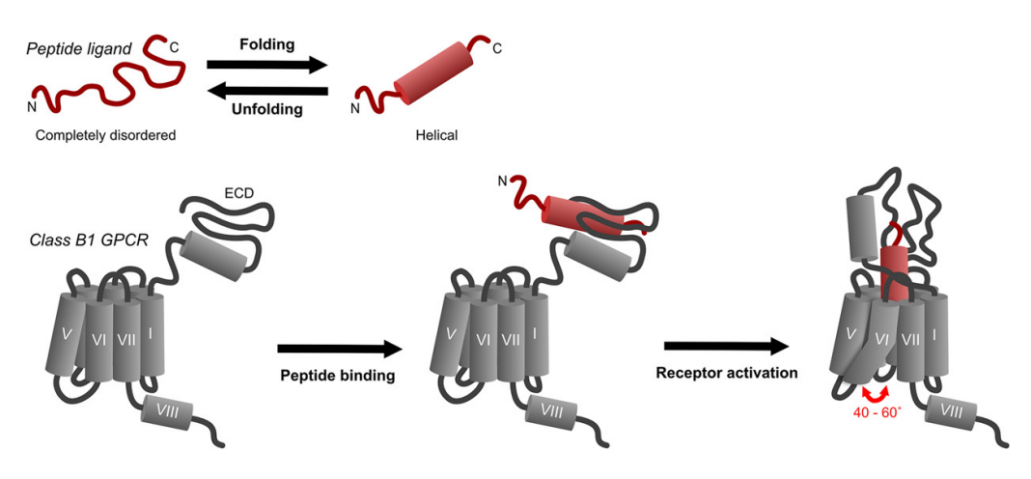
Figure 3 The Generalized schematic showing the binding step and the activation step for a general ligand with the Class B of the G protein coupled receptors
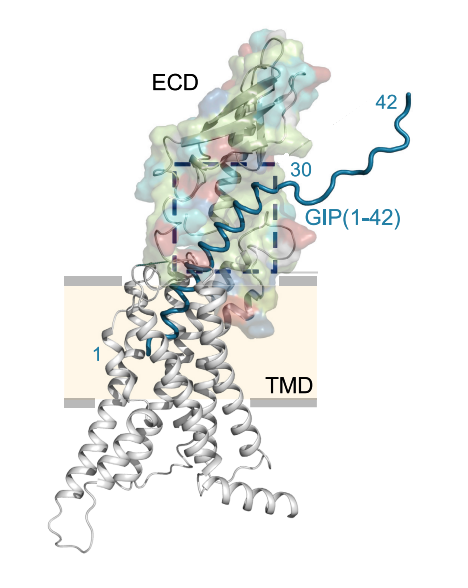
Figure 4 The interaction between Glucose-dependent Insulinotropic Polypeptide GIP which is shown in teal and the GIP Receptor whose transmembrane domains are shown in grey and extracellular domain is shown in multicolor. As it can be seen that the Glucose-dependent Insulinotropic Polypeptide GIP interacts with the extracellular domain as well as with the transmembrane domains of the GIP receptor, but not all of the residual amino acids do interact with the GIP
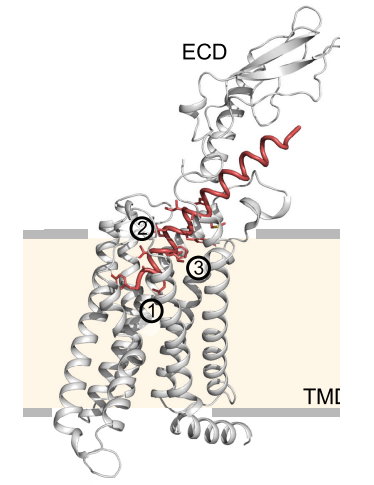
Figure 5 An overview of the three cores of the Hydrogen bonding, which are numbered as 1, 2 and, between the amino acid residues 1-15 of Glucose-dependent Insulinotropic Polypeptide GIP and the transmembrane domain of the GIP receptor
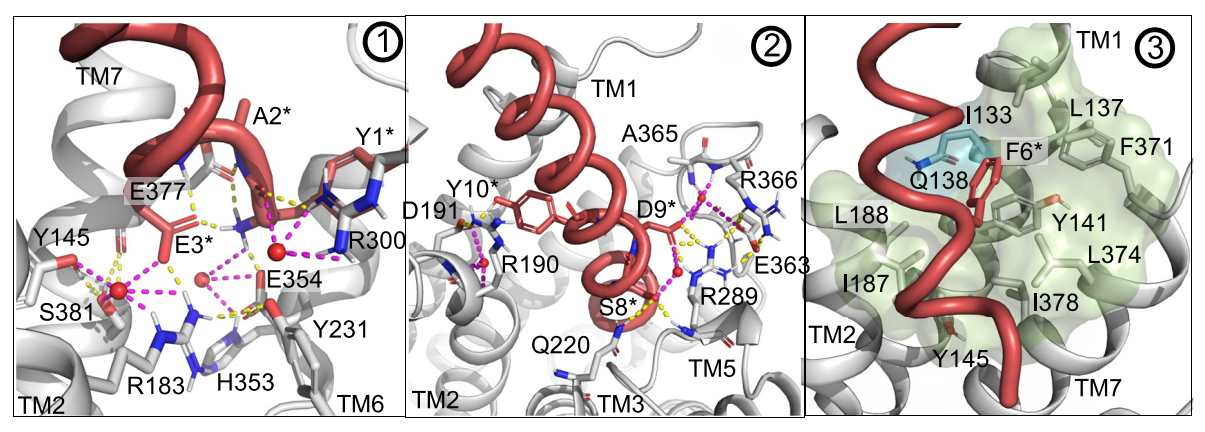
Figure 6 A zoomed in view of the three cores of hydrogen bonding, which are shown in yellow dashes, between the amino acid residues 1-30 of Glucose-dependent Insulinotropic Polypeptide GIP and the transmembrane domain of the GIP receptor. In the part 3, only weak hydrogen bond exists along with some hydrophobic interactions
Activated GIP receptor leads to the activation of a signalling cascade in the cell, resulting in the activation of cyclic adenosine monophosphate cAMP which acts as a second messenger in the cell and the Protein kinase A, increased levels of Calcium ions, followed by the phosphorylation of GIP receptor via the GRKs leading to the recruitment of beta arrestins and causing the internalizing or degradation of the GIP receptor. The activated signalling pathways via the GIP/ GIP receptor are given in Figure 7.
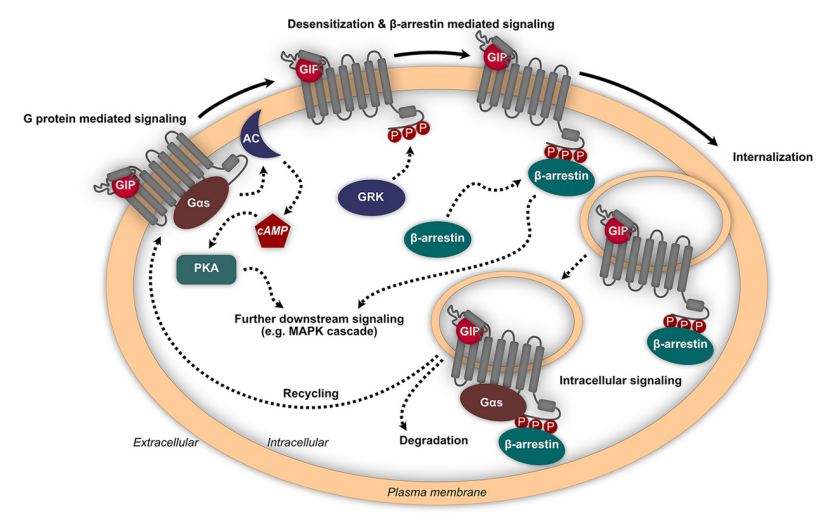
Figure 7 The initiated signaling pathways via the activated GIP receptor





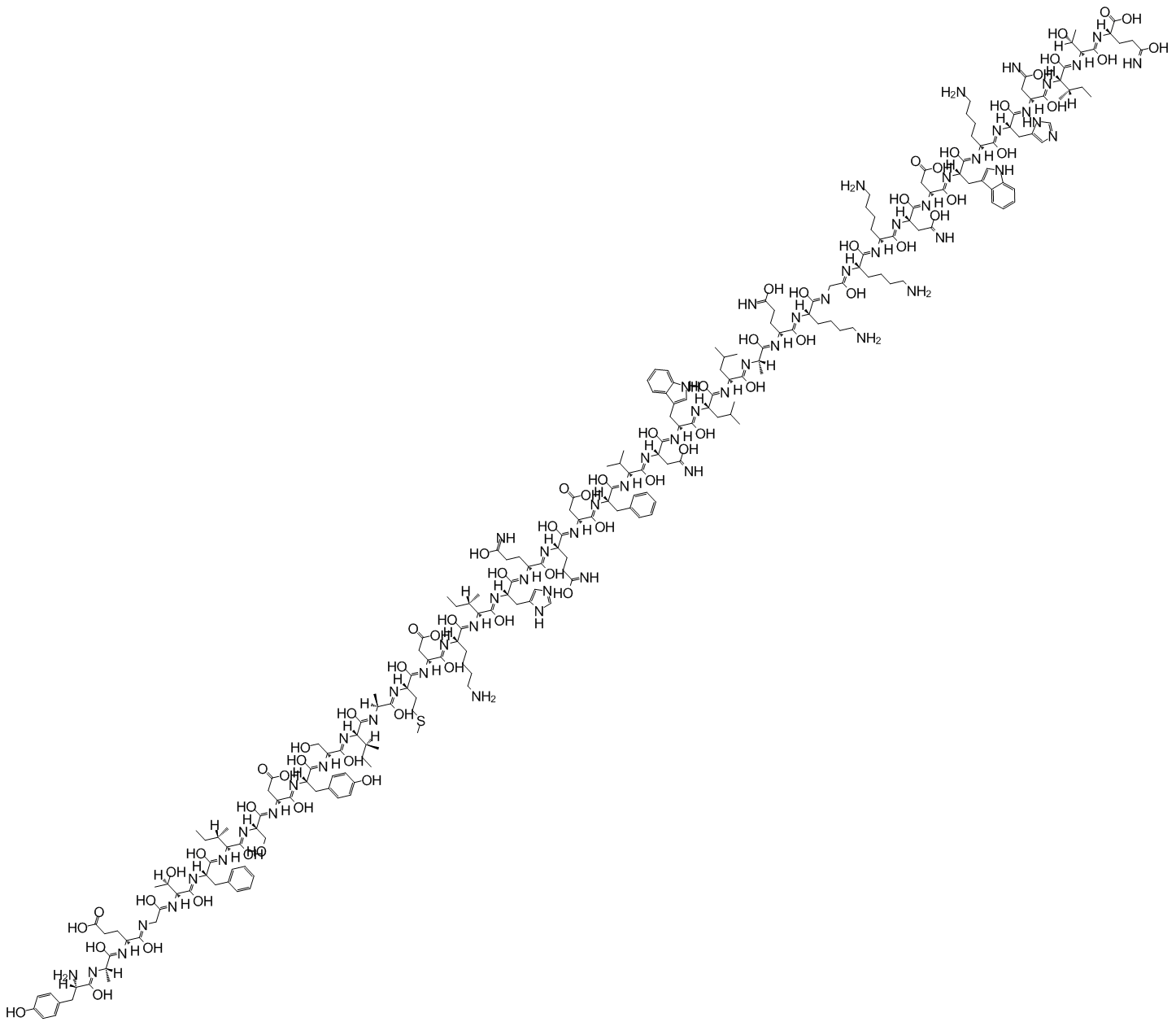








Comments