GW9662
GW9662 exhibits half maximal inhibitory concentration (IC50) of 3.3 nM and is a well-characterized inhibitor of peroxisome proliferator-activated receptor gamma (PPARγ), demonstrating high selectivity for PPARy with the inhibitory activity greater for PPARγ about 10 to 600 times compared to PPARα and PPARδ in cellular environments.
GW9662 is a small molecule with the chemical name of 2-Chloro-5-nitro-N-phenylbenzamide, along with a molecular weight of 276.68 and formula C13H9ClN2O3. With a minimum concentration of 13.75 mg/mL in organic solvents such as dimethyl sulfoxide (DMSO), GW9662 exhibits good solubility, particularly when assisted by sonication along with ethanol (EtOH) at a minimum concentration of 9.08 mg/mL.
It is recommended for optimal storage and use to keep 2-chloro-5-nitro-N-phenylbenzamide at -20°C. A solvent compatible with the desired concentration is selected and sonication is utilized to enhance solubility in a 37°C warm bath for the preparation of stock solutions if necessary. For the maintenance of compound integrity, frequent freeze-thaw cycles should be avoided. To ensure optimal activity, stock solutions should be used within 1 month (-20°C) or 6 months (-80°C) depending on storage and temperature.
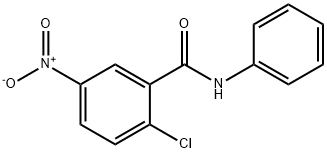
Fig: Chemical Structure of GW9662
Peroxisome Proliferator-Activated Receptors (PPARs): Versatile Regulators in Metabolism and Inflammation,Peroxisome proliferator-activated receptors (PPARs) exist in three isoforms PPARα, PPARβ/δ, and PPARγ, and constitute a subfamily of ligand-activated transcription factors within the nuclear receptor superfamily. These isoforms play a critical role in the regulation of broad-spectrum metabolic pathways and exhibit distinct tissue expression patterns, with significant presence in skeletal muscle, adipose tissue, liver, kidney, vascular endothelium, and intestinal epithelium. The regulation of PPAR activity is done by endogenous ligands, including dietary lipids, phosphatidylcholines, and fatty acids derived from lipolysis.
The pivotal role of PPARy is recognized as regulating genes associated with lipid and glucose metabolism and has also been implicated in modulating inflammatory processes. It has been suggested through randomized clinical trials that for potentially improving hepatic steatosis associated with non-alcoholic fatty liver disease (NAFLD), targeting PPARγ activity with specific agonists like rosiglitazone and pioglitazone can be beneficial. Findings from animal models of obesity and diabetes display elevated hepatic PPARγ levels. The exacerbation of hepatic lipid accumulation might be due to PPARy activation. Liver steatosis might get worse in obese mice with high hepatic PPARγ expression by rosiglitazone treatment. Dampening of hyperglycemia, hyperinsulinemia, and tumor necrosis factor-alpha (TNFα) plasma levels in ob/ob and PPARγ+/− mice and suppression of adipocyte differentiation by PPARγ antagonists is suggested by some other studies supporting this complexity. The PPARγ antagonist GW9662 has been demonstrated to attenuate NAFLD development in ob/ob mice to induce differentiation of macrophages towards an M2c-like phenotype highlighting the multifaceted role of PPARγ in metabolism and inflammation. Continued research will be beneficial to explore the full therapeutic potential of PPARγ agonists as they may offer therapeutic benefits in some contexts, but their effects in others warrant further investigation by presenting a potentially complementary approach.
There is a significant reduction in lipopolysaccharide (LPS)-induced expression of interleukin 1 beta (Il1β), interleukin 6 (Il6), and inducible nitric oxide synthase (iNos), along with a decrease in nitrite (NO2) formation within J774A.1 cell by the administration of GW9662. GW9662 effectively inhibits the development of mammary alveolar lesions (MAL) induced by DMBA. Intriguingly, resveratrol-mediated cell cycle disruption in colon carcinoma cells is prevented by (2.5/5 µM) GW9662 treatment.
Exacerbation of brain damage has been seen in alcohol-fed mice subjected to reperfusion injury by the administration of GW9662 as it appears to have some detrimental effects in certain contexts. Attenuation of renal injury and dysfunction caused by ischemia/reperfusion (I/R) is due to the pre-treatment with LPS, (1 mg/kg; intraperitoneal injection; 24 and 12 hours before ischemia) of GW9662 abolished the protective effects of LPS in model as per the investigation.
PPARγ Antagonist GW9662 Mitigates Diet-Induced NAFLD and TLR4 Signaling, Insulin sensitivity in type 2 diabetes can be improved using GW9662, a potent peroxisome proliferator-activated receptor gamma (PPARγ) antagonist as per some recent studies for non-alcoholic fatty liver disease (NAFLD) aiming to investigate the effects of GW9662 on NAFLD development and the underlying molecular mechanisms of the disease. For eight weeks, female C57BL/6J mice were pair-fed either a control diet or a fat-, fructose-, and cholesterol-rich diet (FFC) while additionally receiving a vehicle administered intraperitoneally or either GW9662 (1 mg/kg body weight) three times a week. Markers for liver damage and inflammation, glucose metabolism parameters, and portal endotoxin levels were assessed in this study, and for further examination of effects, LPS-stimulated J774A.1 macrophages were treated with 10 μM GW9662. Reduced NAFLD development and insulin resistance were displayed by FFC+GW9662-treated mice when compared with the FFC group despite the similar intake of caloric. Higher hepatic expression of toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (Myd88), interleukin 1 beta (IL1β), and nitrite (NO2⁻) concentration was displayed by FFC-fed mice when compared to FFC+GW9662-treated animals. The induction of IL1β, interleukin 6 (IL6), inducible nitric oxide synthase (iNOS), and NO2⁻ formation was significantly attenuated by the GW9662 treatment in LPS-challenged J774A.1 cells.
Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Antagonism and Breast Cancer Cell Growth,To treat obesity, dyslipidemia, and diabetes, a nuclear receptor, Peroxisome proliferator-activated receptor gamma (PPARγ) is targeted by thiazolidinediones. The PPARγ signaling disruption holds promise for cancer therapy, particularly breast cancer as suggested by the emerging pieces of evidence. PPARγ agonists suppress tumor cell growth and survival while tumors exhibit differential PPARγ expression compared to healthy tissues.
effect of GW9662, a potent, irreversible, and selective PPARγ antagonist, on human mammary tumor cell lines effectively blocking PPARγ activation and inhibiting cell growth, was investigated in this study. Results supported the existence of PPARγ-independent pathways in rosiglitazone's anti-tumor effect. Furthermore, the notion that anti-cancer effects are exerted through PPARγ activation by PPARy ligands is challenged. Exploring the potential PPARy independent pathways for breast cancer therapy was the main aim of the research.
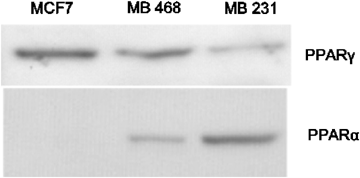
Fig: Expression of PPARγ and PPARα protein in human breast cell lines. Each lane was loaded with 30 μg cell lysate.
Conclusion:
For the investigation of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) specific functions, GW9662 is emerging as a promising tool as it exhibits high binding affinity and functional antagonism specifically towards PPARγ, with minimal effects on PPARα and PPARδ activity. A conserved cysteine residue (Cys285) on PPARγ is covalently modified by GW9662, disrupting its interaction with coactivators and subsequent transcriptional activity as per mechanistic studies. It is a valuable tool for further investigation of PPARγ's specific role in various biological processes because of its selectivity and irreversible nature of GW9662 interaction with PPARγ.














Comments