HBC 620
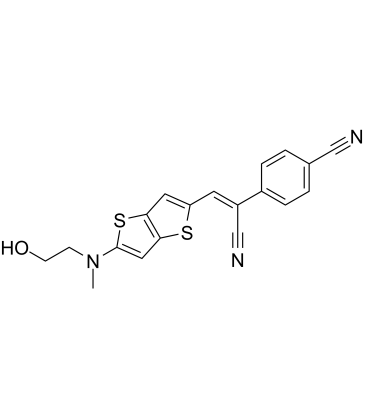
HBC 620 is one of the chemical analogs of HBC. The full chemical name of HBC is (4-((2-hydroxyethyl) (methyl) amino) -benzylidene) -cyanophenylacetonitrile. The 620 in the chemical name of HBC 620 indicates that it exhibits a strong fluorescence at the wavelength of 620 nm when bounded to pepper. Its molecular structure is diagrammatically shown, along with the other HBC analogs of the series, in Figure 1. As evidenced from Figure 1, these analogs of HBC only slightly differ in their molecular structure. This series of the chemical analogs of HBC is generated by the manipulations either in the aromatic π-structure or in the capability of being electron donor and receptor using a methodology that is known as Systematic Evolution of Ligands by Exponential Enrichment (SELEX).
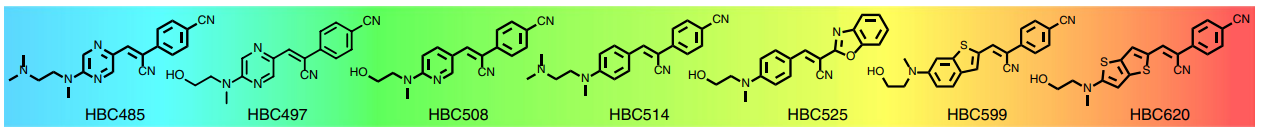
Figure 1 The Chemical Analogs of HBC. The HBC 620 is the rightmost molecule in the series of chemical analogs of HBC. The color in the background of the molecule indicates the region of visible light spectrum in which the emission by the respective molecules lies
HBC 620 is a small, synthetic molecule that is not a fluorophore itself under the normal conditions, i.e., in the solution form. But it does act as a strong fluorophore when it gets non-covalently bounded with a RNA aptamer known as pepper, which essentially means that it absorbs the light radiations having the wavelength lambdapeak = 620 nm and have a bright red color and gets excited (this is shown in red graph in Figure 2) followed by its return to its original state accompanied by the release of visible light radiations having the wavelength lambdapeak = 620 nm and have a bright red color (this is shown in red graph in Figure 3). This release/ emission of light creates a condition that is known as fluorescence. In short, the fluorescence of HBC 620 can be intensified by giving it a treatment with pepper.
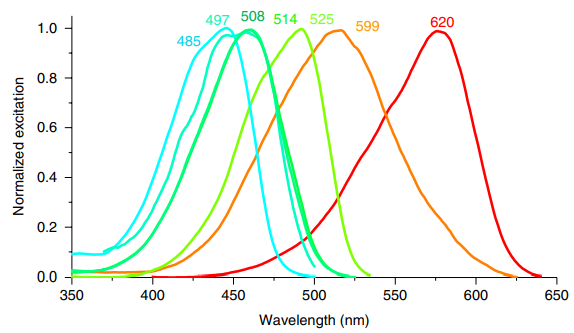
Figure 2 The Excitation Spectrum of HBC 620-pepper complex is shown in Red Graph while other graphs are indicating the excitation spectra of the complexes by other chemical analogs of HBC.
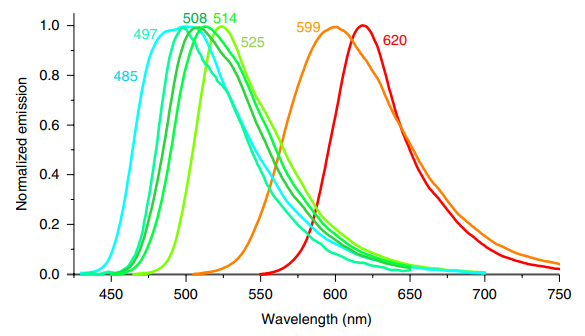
Figure 3 The Emission Spectrum of HBC 620-pepper complex is shown in Red Graph while other graphs are indicating the emission spectra of the complexes by other chemical analogs of HBC. High level of fluorescence brightness as well as high level of fluorescence photostability is associated with the HBC 620-pepper complex. Moreover, red is the most brightest color of the entire spectrum with high visibility.
Pepper belongs to the category of Fluorescent RNAs (FRs). It is an aptamer that is chemically made up of a single strand of Ribonucleic acid RNA molecule; that essentially means that it is composed of ribonucleotides. Structurally, the Pepper molecule is composed of total 49 such ribonucleotides, out of which 43 ribonucleotides are involved in the binding activity, i.e., with the ligand. Its primary, secondary and tertiary structures are diagrammatically shown in Figure 4 and Figure 5. Besides acting as an intensifier for the HBC 620, Pepper is used as a tag/ label for the coding RNA which is mRNA, as well as the non-coding which is RNA ncRNA. These RNAs are called as RNA of interest. Therefore, as the RNA are tagged with this pepper followed by the HBC 620 treatment is widely used to visualize the localization and functions as well as the dynamics of RNAs within the living cell. RNA directly performs a wide range of biological functions in the cell. On the other side, it is also seen that RNA has an indirect role in the maintaining/ regulating each and every function that is taking place in a cell. Therefore, understanding the roles of the RNA in the cell is the pivot of understanding the functioning of the cell. Several different types of RNAs have been studied by using this technique so far namely 5S rRNA, snRNA and splicing RNA. Another key feature of this technique is that there is completely no need of RNA scaffold. The primary reason for this application is the association of high brightness and photostability with the complex formed between pepper and HBC 620. This concept is schematically depicted in Figure 6. This concept can be thought as a FR/dye system in which maximum brightness is attained when pepper and HBC 620 for a mutual molecular complex. This is evident from the given matrix in Figure 8 which is further based on the fluorescence observed in the live cells that is pictorially presented in Figure 9. Moreover, the graphical representation of this same data samples is given in Figure 10.
Pepper is a monomeric that generates multicolor fluorescence depending upon the chemical nature of its ligand. But with HBC 620, it creates a bright red color fluorescence. It has greater affinity for its ligands, higher melting point and a high tolerance against a high range of pH. High melting point indicates that it is a thermostable compound.
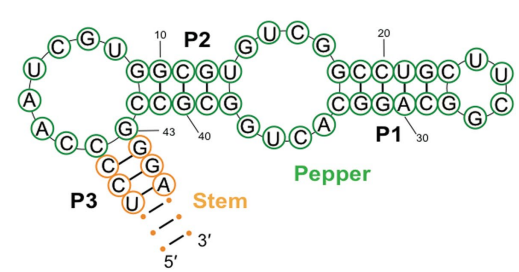
Figure 4 The Primary Structure and Secondary Structure of the Pepper is shown by the letter representation of the amino acids and the Pin numbers, respectively.
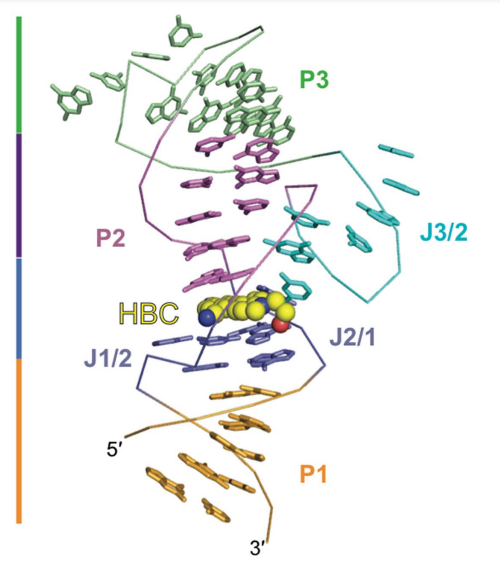
Figure 5 The Tertiary Structure of the Pepper as estimated through the X-ray crystallography, that is non-covalently bound to the HBC. Here, HBC can be any of its chemical derivatives. Also, Stems Pin 1, Pin 2, Pin 3 and the junctions J1/2 and J2/1 constitute a helical structure.
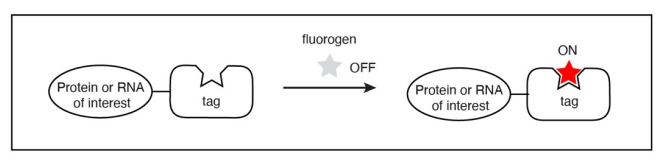
Figure 6 The main concept of using tags/ labels which are both RNA binging as well as acting as a molecular switch for the fluorogen molecule.
In the case of HBC 620, a bithiophene having a longer length was introduced in the structure of HBC (as indicated in red color in Figure 7) in order to improve the conjugation of the pie electrons. As HBC 620 has longer length, no hydrogen bond can form between HBC 620 and pepper. This observation is explained in Figure 7. Moreover, the interactions by which the whole complex is stabilized are highly reversible in nature.
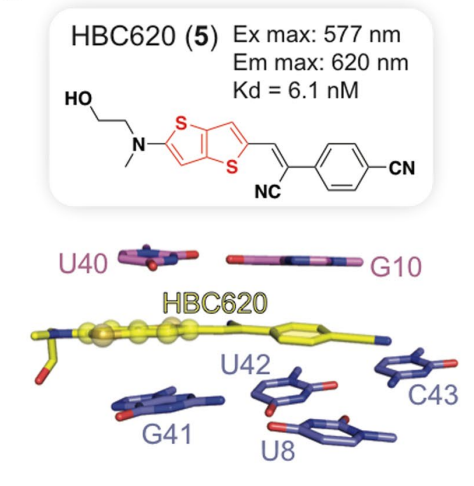
Figure 7 The introduced bithiophene motif in the HBC Structure. In the bottom, only those bases of the pepper are shown which are interacting with the HBC 620 molecule. As it can be seen that no hydrogen bonds are present in HBC 620-pepper complex
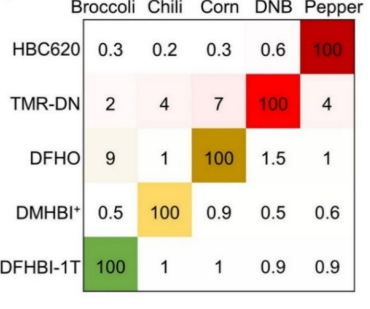
Figure 8 The derived matrix showing the maximal brightness that is given by the complexes of fluorogen and the nonfluorescent molecule or FR/dye systems.
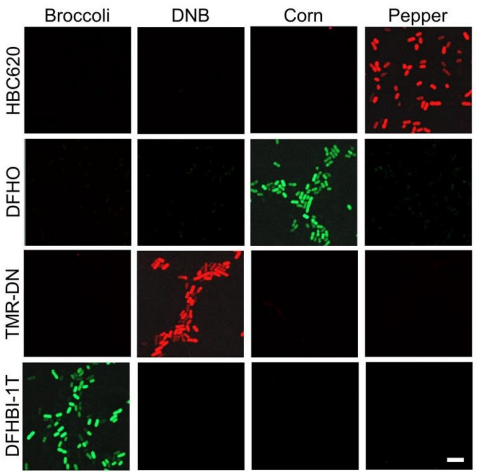
Figure 9 The different intensity levels of the fluorescence that is exhibited by the different complexes or FR/dye systems
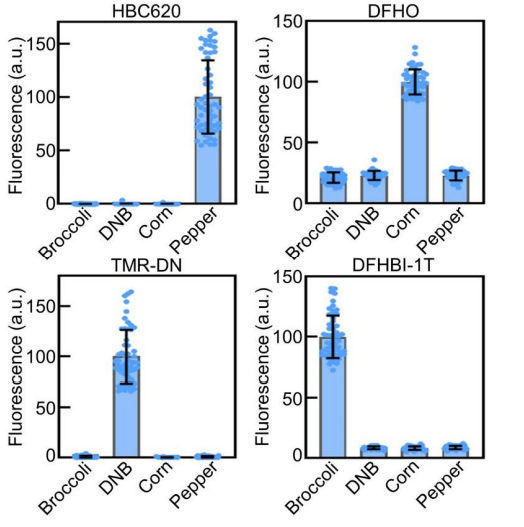
Figure 10 The graphical presentation of the different intensity levels of the fluorescence that is exhibited by the different complexes or FR/dye systems













Comments