IraZolve-Mito
With a high selectivity for mitochondria in living cells, IRAZOLVE-MITO (iridium complexed with cyclometalated 2-phenylpyridine and the 5-(5-(4-cyanophen-1-yl) pyrid-2-yl)tetrazolate ligand) exhibits an emission spectrum in the 505–625 nm range 22. In contrast to organic fluorophores, they have extended excited state lifetimes ranging from hundreds of nanoseconds to milliseconds, are resistant to photobleaching, and penetrate the cell membrane quickly 23. In live cells and tissues, IraZolve-Mito localizes to the mitochondria. For various types of fixed and frozen tissue sample mitochondria detection, IraZolve-Mito is also appropriate. Numerous fluorescent applications, such as multiphoton and confocal microscopy imaging, are appropriate for this agent. IraZolve-Mito's cell penetration and localization have been verified in cancer cell lines (HeLa), cardiomyocytes (H9c2), and prostate cells (PNT2 and 22RV1). Live tissues, such as sheep adipose and skeletal muscle, as well as frozen or PFA-fixed muscle, have all been demonstrated to have mitochondrial localization (sheep skeletal). The molecular structure of Irazolve-mito is shown in Figure 1.
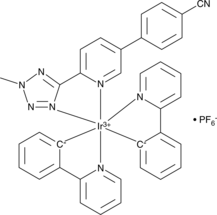
Figure 1: The molecular structure of IraZolve-Mito.
Small fluorescent compounds and fluorescence imaging via immunochemistry are the main tools used in mitochondrial imaging . Because of the organelles' transmembrane potential, organic fluorophores—which make up the bulk of commercially available mitochondrial dyes—accumulate in the mitochondrial matrix. As a result, these dyes should only be applied to living samples, such as JC-1and the MitoTrackers CMTMRos and CMXRos . Immunochemistry has been the primary method used to visualize mitochondria in fixed materials up to now. Antibody detection is highly sensitive, however it takes a long time and involves several processing steps that could result in considerable artifacts. Furthermore, a variety of model animals may be unable to utilize them due to problems with antibody availability. Small molecule imaging instruments that can swiftly and efficiently image mitochondria in both live and fixed tissue samples are thus required . The iridium compound, marketed under the name IraZolve-Mito, is highly selective for mitochondria in living H9c2 rat cardiomyoblasts . When compared to organic fluorophores, iridium complexes and other transition metal complexes have better photostability, larger Stoke shifts, and longer excited states, which has led to a growing interest in their application as imaging agents. Although a number of iridium complexes have been shown to localize to mitochondria their applicability for mitochondrial detection in fixed tissue samples has not yet been investigated. We have validated a procedure for the imaging of mitochondria using IraZolve-Mito in both live and fixed tissues by confocal microscopy, in response to the requirement for novel mitochondrial stains that are compatible with fixed tissue samples.
IraZolve-Mito localization was evaluated in fresh frozen, fixed, and living muscle tissues. Because of their high mitochondrial abundance and clinical importance for altered mitochondrial morphology, muscle tissues were chosen. Samples of tissue were taken from the adult sheep's quadriceps (skeletal muscle) and left ventricle (cardiac muscle). Tissue samples were cut into approximately 5-mm cubes for live imaging, and they were incubated with 20µM of IraZolve-Mito in 0.2% (v/v) DMSO/PBS for 30 minutes at room temperature. Following this incubation period, the tissues underwent a 5-minute wash before being mounted and examined using a Nikon A1+ confocal microscope. As previously mentioned, IraZolve-Mito displayed a broad emission band with an emission maxima at around 608 nm when stimulated at 403 nm . Consequently, images were captured in the emission range of 505–625 nm. IraZolve-Mito stained cylindrical organelles (Fig. 2) that resembled mitochondria in vivo cardiac and skeletal muscle. These organelles were found within the muscle fibers in a regular network structure (Fig. 2a,c) and in close proximity to sarcomeres (Fig. 2). This unique distribution aligned with earlier findings about mitochondria in skeletal muscle .
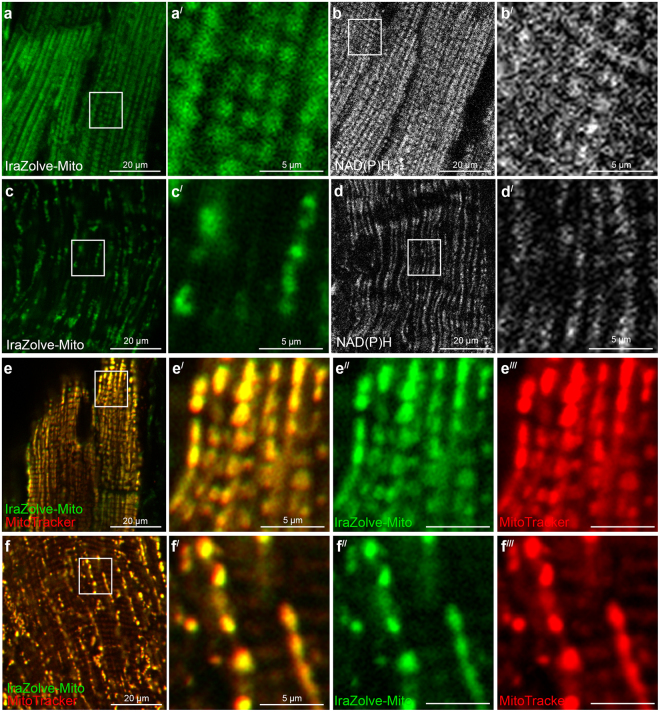
Figure 2: IraZolve-Mito detects mitochondria in live cardiac and skeletal muscle samples. Representative confocal micrographs showing mitochondria detected with IraZolve-Mito in cardiac (a) and skeletal (c) muscle samples.
Both cardiac muscle (Pearson's correlation 0.84 ± 0.01, n = 6; Fig. 1e–e) and skeletal muscle (Pearson’s correlation 0.86 ± 0.03, n = 7; Fig. 1f–f) showed a significant co-location of these dyes, supporting the previous observations in live cells that IraZolve-Mito is localized to mitochondria .
Using preserved cardiac and skeletal muscle tissues, IraZolve-Mito staining is performed. Following about 20 hours of fixation in 4% paraformaldehyde (PFA) and a 30-minute wash phase, the tissues were kept in PBSF at 4 °C. The same methodology that was optimized for living tissues was used to these fixed samples for IraZolve-Mito staining and visualization. The staining pattern of IraZolve-Mito in fixed tissue (Fig. 3a, c) was found to be identical to that of living muscle samples (Fig. 3a, c). Additionally, the staining pattern matched that of antibody probing for the cytochrome C protein, which is localized in the mitochondria's inner membrane (Fig. 3b,d). To ensure accuracy, live tissue samples were first stained with MitoTracker and then counter-stained with IraZolve-Mito following fixation in 4% PFA. In order to prevent MitoTracker from being lost during this operation, the tissue fixation was done for one hour, yet this was enough time to fix these tiny samples (thickness of less than five millimeters). IraZolve-Mito's ability to imaging mitochondria in PFA preserved tissue was confirmed by the co-location of MitoTracker and IraZolve-Mito in fixed cardiac muscle (Pearson's correlation 0.89 ± 0.01, n = 4; Fig. 3e) and skeletal muscle (Pearson’s correlation 0.89 ± 0.02, n = 3; Fig. 3f).
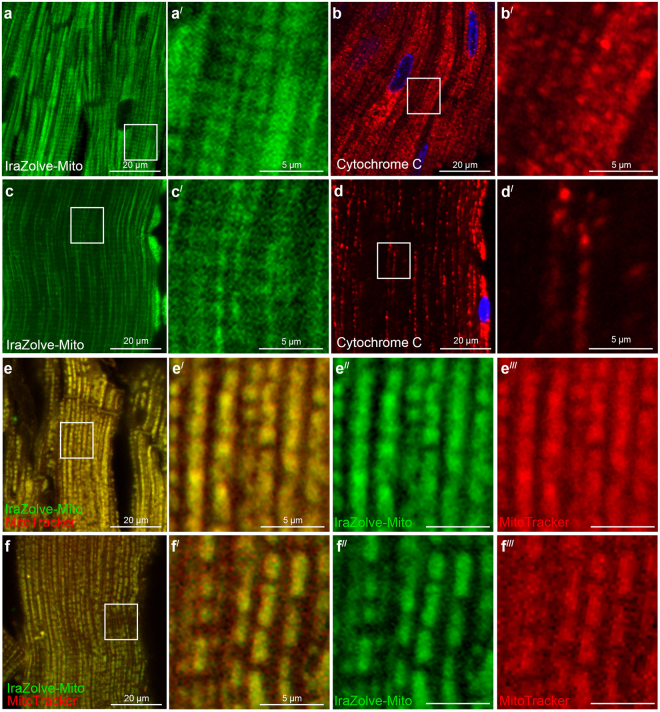
Figure 3: Staining pattern of IraZolve-Mito is maintained in fixed cardiac and skeletal muscle samples. Representative confocal micrographs showing subcellular distribution of IraZolve-Mito in 4% PFA fixed cardiac (a) and skeletal (c) muscle samples.
The fact that IraZolve-Mito is compatible with mitochondrial labeling of fixed tissue indicates that its cationic character is not the only mechanism governing localization. In order to verify that the location of IraZolve-Mito within mitochondria was independent of membrane polarization, we employed carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP)20 to de-regulate the mitochondrial membrane potential. The FCCP treatment of H9c2 rat cardiomyoblasts did not affect the IraZolve-Mito mitochondrial labeling. In contrast, cells treated with FCCP showed diffuse cytoplasmic staining according to MitoTracker, which is reliant on mitochondrial membrane potential8,9. (Fig. 3). Following FCCP treatment, morphological alterations to mitochondria were validated by Cytochrome C immunofluorescence, which matched IraZolve-Mito labeling patterns (Fig. 3c,g). This suggested that mitochondrial accumulation with IraZolve-Mito labeling did not depend on membrane polarization.
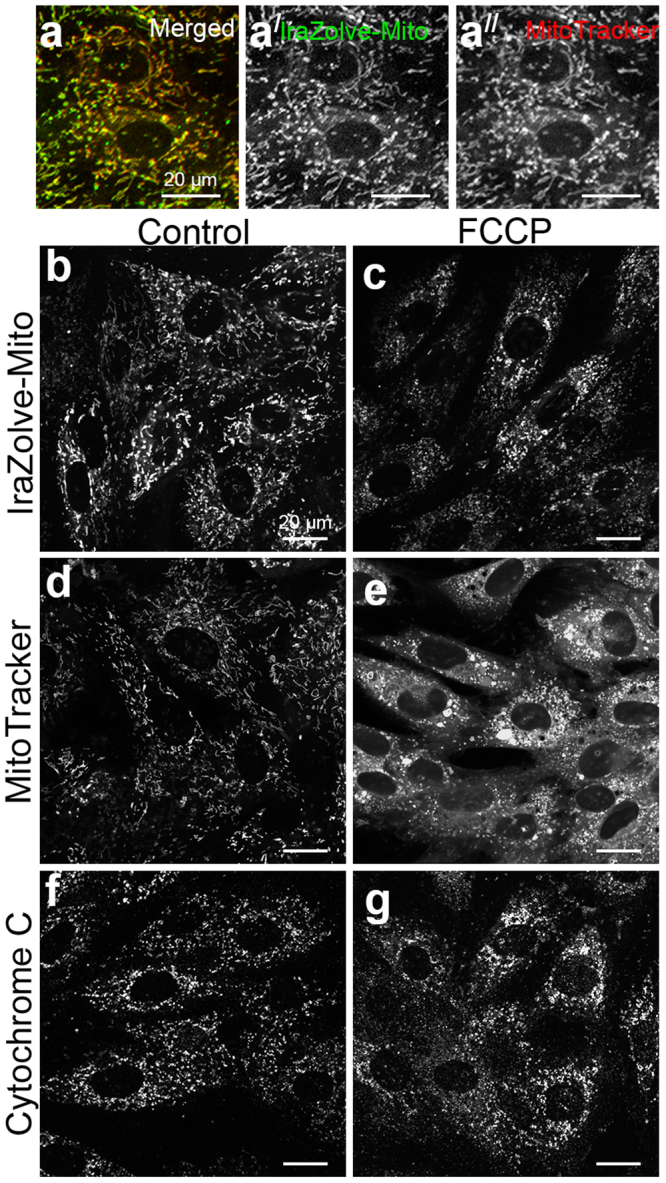
Figure 4: Inhibition of mitochondrial membrane potential in H9c2 rat cardiomyoblasts does not disrupt IraZolve-Mito staining. (a) Micrographs showing co-staining of IraZolve-Mito (green in a; greyscale) with MitoTracker Red CMXRos in cardiomyoblasts.
To the best of our knowledge, IraZolve-Mito is the first compound of its kind for a tiny molecule with the capability to easily enter and image mitochondria in fixed tissues. This ability to picture mitochondria in both live and fixed tissues makes the compound unique. IraZolve-Mito is widely applicable to tissue samples, even in settings of mitochondrial stress that may produce membrane depolarization or protein relocation, because the localization is not dependent on membrane potential or protein localization. The ability to stain fixed tissue also gives researchers a simple substitute for antibody staining that doesn't need them to think ahead and stain live tissues while they are being collected. Researchers now have more flexibility in experimental design and sample preparation thanks to the identification of small luminescent molecules like IraZolve-Mito, which is ideal for imaging mitochondria in fixed tissue samples as well as live cell and tissue imaging. This also eliminates the need for multiple reagents.













Comments