Netarsudil mesylate
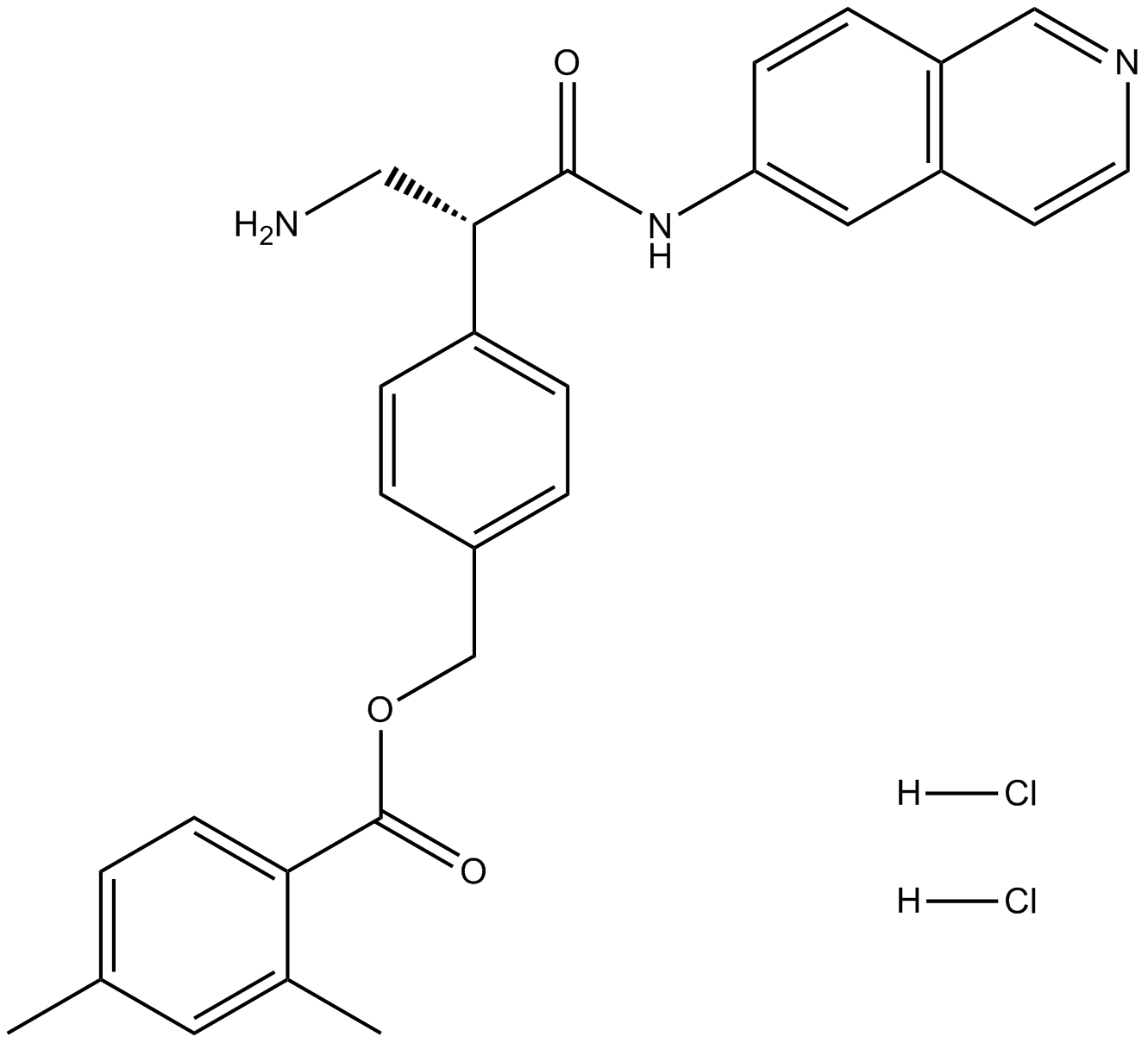
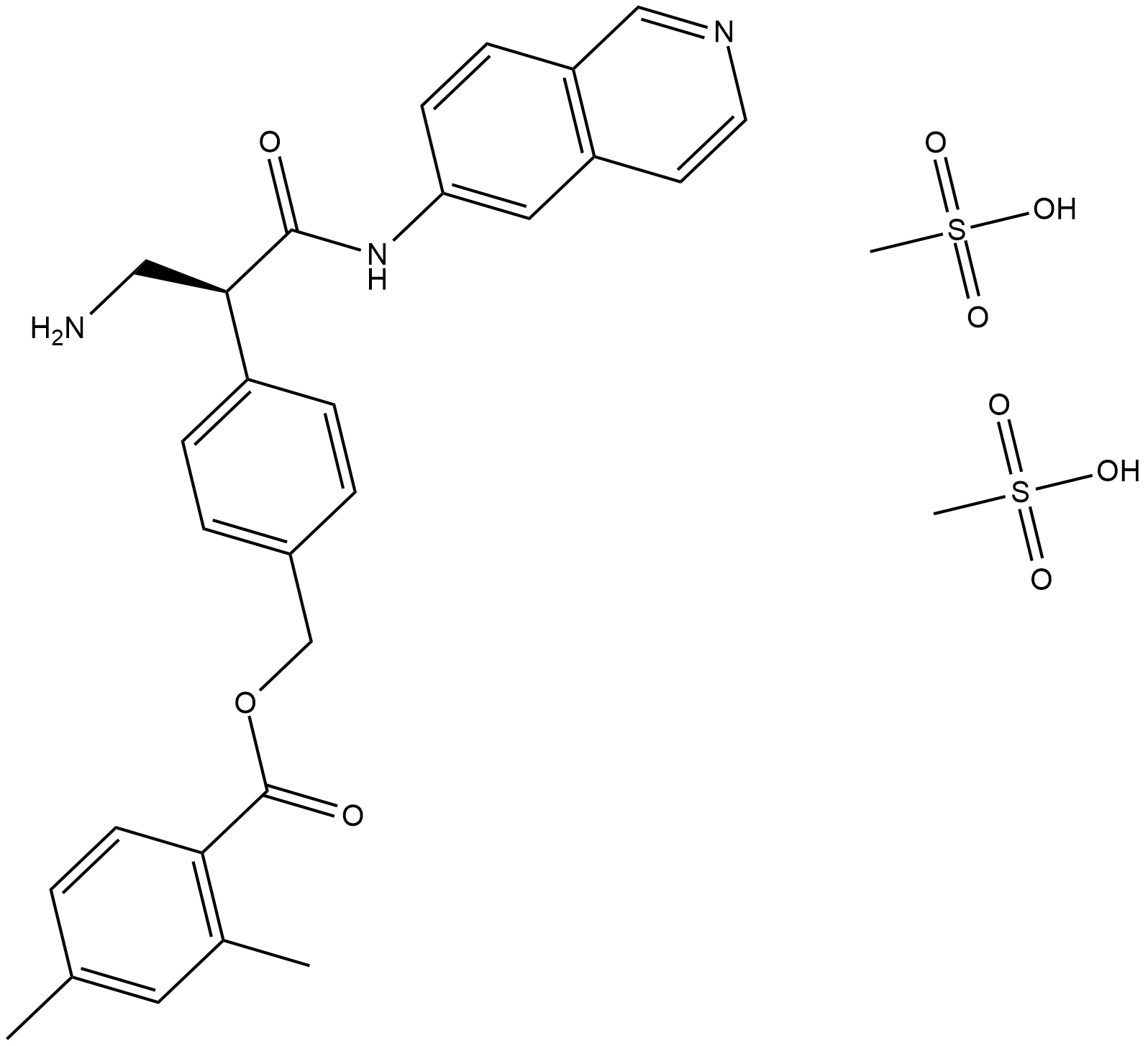
AR-13324 is also known as Netarsudil dimesilate or Netarsudil mesylate. Chemically, AR-13324 is a an amino isoquinoline amide that is a member of Mesylates, which were first identified as the most effective ROCK inhibitors with high tolerance and having a long-lasting decrease in the intraocular pressure (IOP). Thus, it was selected for clinical development and more research studies were conducted using AR-13324. The two dimensional view of the molecular structure of AR-13324 is given in Figure 1. In 2017, FDA finally approved AR-13324 as an ophthalmic solution of 0.02% concentration for the clinical treatment of glaucoma in humans. It is an optimized formulation of AR-13324 with high levels of safety and efficacy associated for therapeutic use by human.
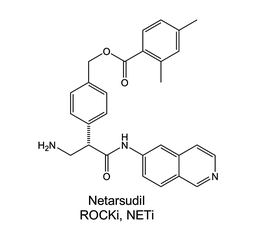
Figure 1 The two dimensional view of the Molecular structure of AR-13324
PHARMACEUTICAL ACTION
It is a new and well-known inhibitor of rho-associated protein kinase (ROCK) and norepinephrine transporter (NET). Inhibiting ROCK decreases the molecular contraction of actomyosin in smooth muscle cells and also in the smooth muscle-like cells of the trabecular meshwork. ROCK is a serine/threonine kinase protein, that is an important effector protein that works downstream of Rho GTPase. There are two isoforms of ROCK, i.e., ROCK 1 and ROCK 2, both have an overall 65% similarity with respect to their molecular structures, while both also have 87% similarity with respect to the structure of their kinase domain. That is the reason that AR-13324 can interact and inhibit ROCK 1 as well as ROCK 2. Taking a closer look at the structure of these two isoforms of ROCK, both have a kinase domain at the N-terminal which is capable to phosphorylate target molecules, particularly proteins, then both have a coiled-coil region that is capable to bind to Rho, after that both have a domain that is similar in structure to pleckstrin, and then finally both have a cysteine-rich autoinhibitory domain at the C terminus that is capable to control kinase activity via intramolecular interactions. The transition between the ON and OFF states of the ROCK molecule is explained via the schematic diagram in Figure 2. The molecular activity of ROCK in both, the trabecular meshwork (TM) and the Schlemm's canal cells, leads to the contraction of actomyosin, a promotion of the extracellular matrix production, and an increment in the overall cell stiffness. While, on the other hand, inhibitors of ROCK leads to the reduction in the contraction of actomyosin, a demotion in the expression of fibrosis-related proteins in the extracellular matrix, and a decrement in the overall cell stiffness.
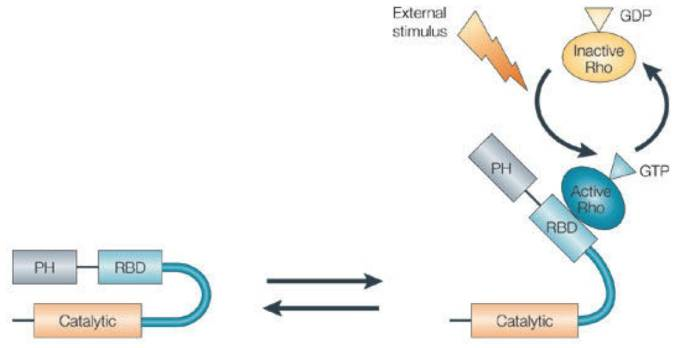
Figure 2 The ON and OFF states of ROCK. The Schematic Diagram of the ROCK, which is same for ROCK 1 and ROCK 2. In the left side, there is a schematic diagram of ROCK in inactive form due to the formation of a loop via the intramolecular interactions. In the right side, as GTP interacts with Rho and thereby activates it, the active Rho gets bound to the RBD of the ROCK. As this binding happens, the inhibitory loop of the ROCK molecules breaks and the ROCK becomes active.
THERAPEUTIC ACTION
It is a novel potential pharmaceutical drug for lowering the intraocular pressure (IOP), this pharmaceutical action would have a clinical significance for the potential treatment of eye diseases, namely, glaucoma and ocular hypertension, etc. which are the most common leading cause of blindness in the whole world. It does so by increasing the aqueous outflow through the trabecular meshwork (TM). On the cellular and tissue level, glaucoma is a pathological condition of the optical nerve that marked by the excavation of the optical nerve and the apoptosis in the ganglion cells of the retina. Interestingly, lowering the intraocular pressure is the only well-established intervention against the treatment of blindness due to glaucoma. In an animal model, especially a Dutch Belted (DB) rabbit model, based research study has shown that has caused a decrease in the episcleral venous pressure (EVP). In addition, AR-13324 has also been shown to reduce the production of the aqueous humor (AH) in the monkeys. Both of these observations are proposed to be caused due to the inhibition of norepinephrine transporter (NET) via the activity of AR-13324. However, a research study has been conducted on the donor human eyes; in that study it was observed that an increase in the trabecular outflow facility occurred via an increase in the effective filtration area of the trabecular meshwork (TM), expansion of the trabecular meshwork (TM) tissue, and dilation of the episcleral veins.
Under the normal physiological condition, the aqueous humor (AH) drains majorly through the trabecular outflow. The intraocular pressure (IOP), as the name suggests, is the pressure level at which the aqueous humor is being produced in the ciliary body and is being flowing into the posterior chamber of the eye, in the eye. The conventional path for the aqueous humor (AH), includes trabecular meshwork, Schlemm’s canal, aqueous veins and collector channels) and unconventional path includes the uveoscleral and uveovortex pathways. The intraocular pressure (IOP) is normally regulated within an extremely narrow physiological range via the outflow of the aqueous humor (AH); which is primarily pressure sensitive in nature. Under the abnormal pathological condition, for example an eye with glaucoma, the intraocular pressure (IOP) is abnormally elevated; this elevation is caused due to the presence of high resistance that is offered to the outflow of the aqueous humor (AH) via the trabecular meshwork. Although, the actual underlying cause of this increase resistance is not known; but apparently increased contractile tone and stiffness of the trabecular meshwork (TM), modifications in the extracellular matrix deposition and permeability of the inner wall of Schlemm's canal seem to be associated with the increased resistance to the outflow of aqueous humor (AH).
AR-13324 causes the disrupture of the actin stress fibre and the focal adhesion between the cell; both of these effects are pictorially as well as graphically presented as a function of dose/ concentration of AR-13324 in Figure 3, Figure 4, Figure 5 and Figure 6. Moreover, AR-13324 causes the inhibition of the production of fibres in the extracellular matrix. This phenomenon is pictorially presented in Figure 7.
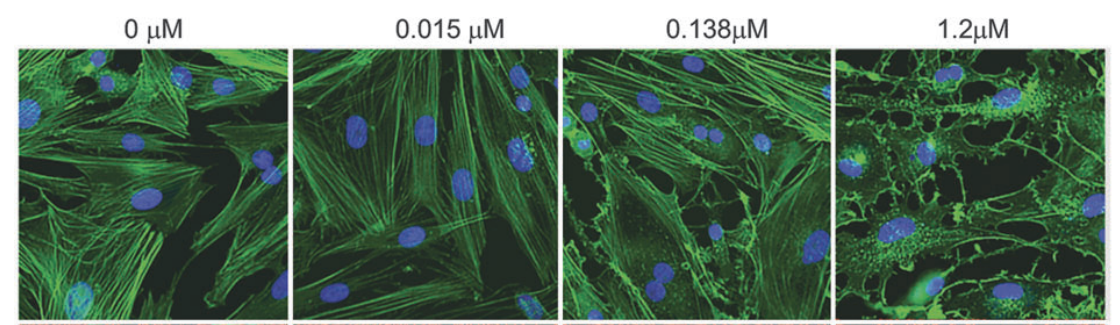
Figure 3 The Effect of AR-13324 dose/ concentration on the actin stress fibre. At higher AR-13324 dose/ concentration, the actin fibres are greatly stressed out and resultantly the cell shape is compromised.
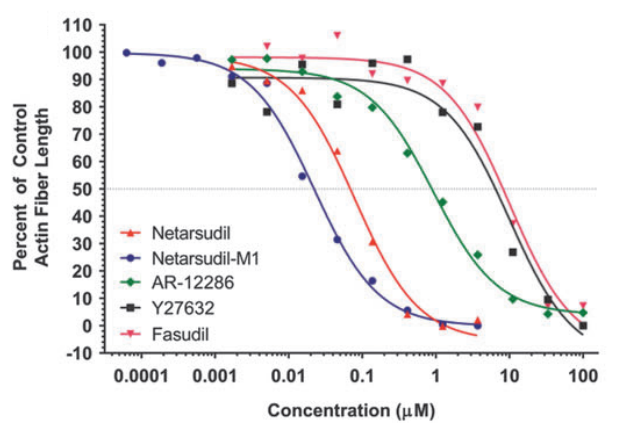
Figure 4 The Graphical Representation of the Effect of AR-13324 dose/ concentration on the actin stress fibre. The upright triangle is the legend for AR-13324
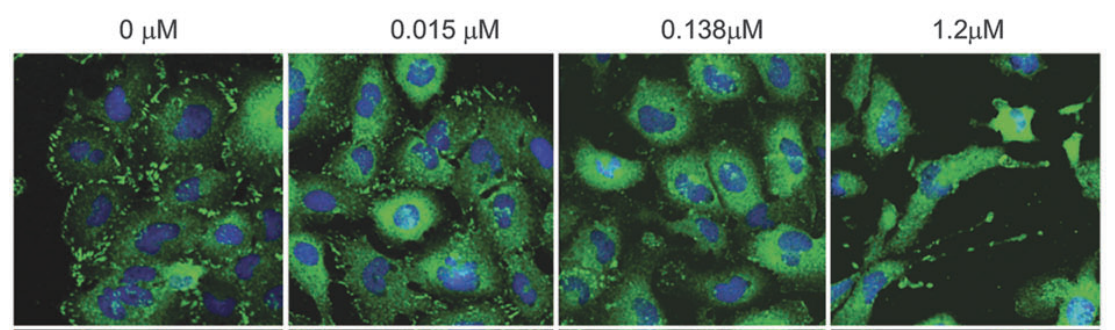
Figure 5 The Effect of AR-13324 dose/ concentration on the focal adhesion between the adjacent cells. At higher AR-13324 dose/ concentration, the focal adhesion is dramatically disturbed, resultantly the cell-to-cell communication is greatly disturbed
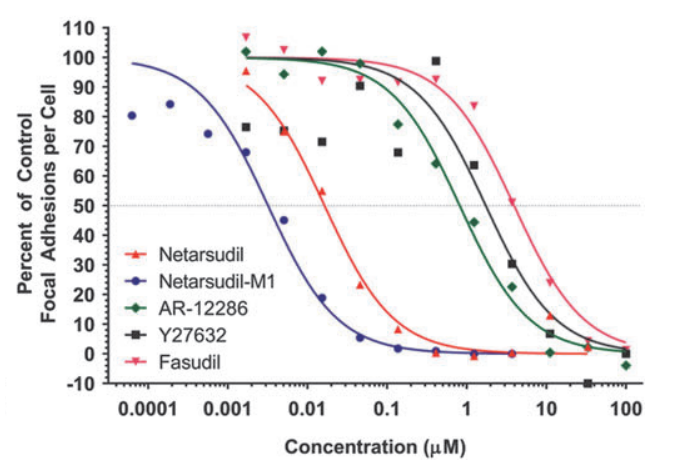
Figure 6 The Graphical Representation of the Effect of AR-13324 dose/ concentration on the focal adhesion between the adjacent cells. The upright triangle is the legend for AR-13324
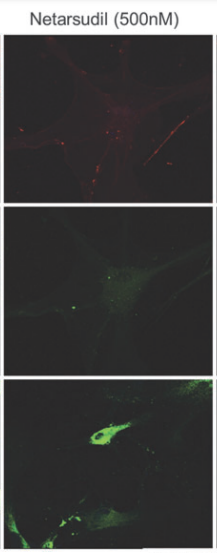
Figure 7 Inhibition of Fibrosis, i.e., formation of fiber by the extracellular matrix, via the action of of AR-13324













Comments