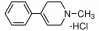
Phos-tag biotin BTL-104
A monobiotinylated Phos-tag derivative called BTL-104 is used to identify phosphopeptides and phosphoproteins. When the ligand molecule and biotin combine to form a functional conjugate (Phos-tag Biotin BTL-104), phosphorylated analytes on a PVDF membrane can be seen, making Phos-tagTM a potent tool. The technique works well for phosphoprotein identification in Western blot analysis without the need for an antibody. Phos-tagTM's chelating action is the only factor that determines the conjugate's affinity for phosphorylated molecules, allowing for a sensitivity of about 1 ng per band. In a second stage, streptavadin-conjugated horseradish peroxidase (HRP) and chemiluminescent detection reagents are used to visualize the bands. When utilizing an anti-serine/threonine phospho antibody is challenging, PhosTag-Biotin might be utilized as an alternative to an antiphospho antibody. It is helpful for SPR investigations, separation/immunoprecipitation, and a range of immunoassay techniques. . Structure of Phos-tag biotin BTL-104 is shown in figure 1.
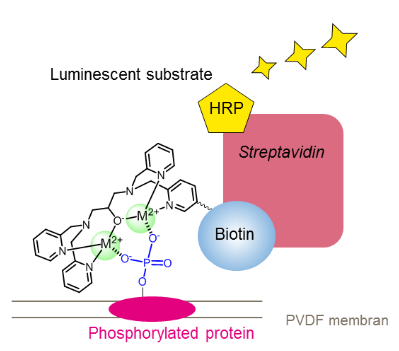
Figure 1: Structure of Phos-tag biotin BTL-104.
Kinase-induced phosphorylation and phosphatase-induced dephosphorylation are processes that activate and deactivate proteins, respectively, that are involved in essential physiological processes. Dysregulation of kinase and phosphatase activity results in a wide range of illnesses. Numerous kinase anomalies have been linked to neurological diseases and malignancy. Thus, the study of protein phosphorylation is essential because it can help discover the underlying causes of a number of illnesses and facilitate the creation of kinase inhibitors, or molecules that target specific proteins. Thus far, we have created methods for analyzing protein phosphorylation through the use of Phos-tag, a useful molecule that selectively binds to a phosphate group (-PO32-). Three Phos-tag-based methods for life science researchers are presented in this article .
In applications like western blotting or microarray-based techniques for thorough identification of phosphorylated proteins, phospho-tag biotin can be employed as a molecular tool. This method involves the formation of a compound in a 4:1 ratio between Phos-tag Biotin and a streptavidin tetramer bound by horseradish peroxidase (HRP), which is then followed by probing of the blot membrane or microarray. When the chemiluminescence (ECL) substrate reacts with HRP, phosphorylated proteins that are selectively linked to Phos-tag Biotin are identified as increased ECL signals (Figure 2).
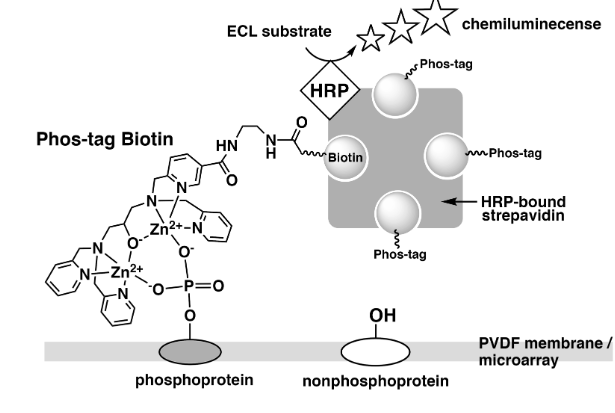
Figure 2: Detection of phosphorylated proteins on PVDF membrane or microarray using Phos-tag Biotin.
We utilized an antibody microarray Proteome Profiler (Human Phospho-MAPK Array, R&D Systems) that is commercially available to evaluate phosphorylated proteins utilizing Phos-tag Biotin in conjunction with the ECL system. 5) The 52 distinct phosphorylation-independent capture antibodies used in this microarray are each spotted in duplicate on a nitrocellulose membrane. We looked at the phosphorylated proteins in two Raw 264.7 cell lysate samples both before and after they were treated with PMA and lipopolysaccharide (LPS) (Figure 3). Using the antibody microarray technique with Phos-tag Biotin, we verified the considerable rise in the levels of phosphorylation of various proteins, including Akt2, HSP27, and p38b, which is consistent with the findings of several earlier studies on LPS/PMA signaling. This method enables the simultaneous assessment of several proteins' phosphorylation state in a lysate sample .
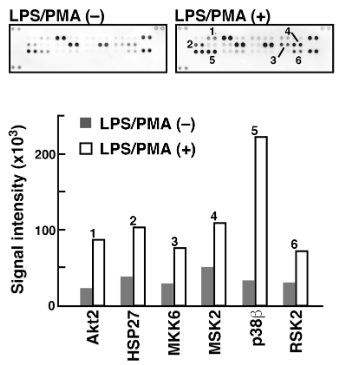
Figure 3: Detection of phosphorylated proteins in Raw264.7 cells before and after the stimulation with LPS/PMA
We assessed the potency of biotin-dependent Zn2+-Phos-tag (refer to Fig. 4) as a phosphate-binding biotin derivative for Western blotting analysis in order to visualize phosphorylated proteins. In the initial instance, phosphorylated proteins (namely, α-casein, β-casein, ovalbumin, and pepsin) spotted on a PVDF membrane were precisely identified at nanogram levels by the use of an ECL apparatus in conjunction with a 4:1 complex of biotin-dependent Zn2+-Phos-tag and HRP-SA, all without the need for membrane blocking. Fig. 4a displays a typical ECL image obtained using dot-blotting technique. The equivalent dephosphorylated and nonphosphorylated proteins (i.e., carbonic anhydrase, β-galactosidase, bovine serum albumin, and human serum albumin) did not exhibit any ECL signal on their spots. No ECL signals were found on the spots of phosphorylated proteins in the absence of the zinc(II) ions (i.e., using a 4:1 complex of biotin-dependent Phos-tag ligand and HRP-SA in the presence of 1 mm EDTA) (data not shown). Therefore, the complex of biotin-dependent Zn2+-Phos-tag and HRP-SA generated the phosphate-selective ECL signals by the interaction of the zinc(II) ions with the phosphomonoester dianion.
Following SDS-PAGE, we subjected the biotin-dependent Zn2+-Phos-tag and HRP-SA complex to an electroblotting examination. This application did not call for a PVDF membrane to be blocked. On PVDF membranes, it was possible to see the phosphorylation of Abltide-GST incubated with tyrosine kinase Abl and the dephosphorylation of phosphorylated Abltide-GST incubated with tyrosine phosphatase TC-PTP. A GST-tagged Abl substrate peptide (Abltide, Glu-Ala-Ile-Tyr-Ala-Ala-Pro-Phe-Ala-Lys-Lys-Lys) is fused together to form the recombinant fusion protein known as Abltide-GST. Abl preferentially phosphorylates the Tyr 4 residue (italicized) of the Abltide segment. Figure 4b and c depict the time histories of the phosphorylation and dephosphorylation, respectively.
We performed an electroblotting analysis on the biotin-dependent Zn2+-Phos-tag and HRP-SA complex after SDS-PAGE. It was not necessary to block a PVDF membrane for this application. Phosphorylation of Abltide-GST incubated with tyrosine kinase Abl and dephosphorylation of phosphorylated Abltide-GST incubated with tyrosine phosphatase TC-PTP were seen on PVDF membranes. The recombinant fusion protein known as Abltide-GST is created by fusing together an Abl substrate peptide (Abltide, Glu-Ala-Ile-Tyr-Ala-Ala-Pro-Phe-Ala-Lys-Lys-Lys) that has been GST-tagged. Tyr 4 (italicized) in the Abltide segment is the specific residue that Abl preferentially phosphorylates. The temporal histories of the phosphorylation and dephosphorylation are shown in Figures 4b and c, respectivel .
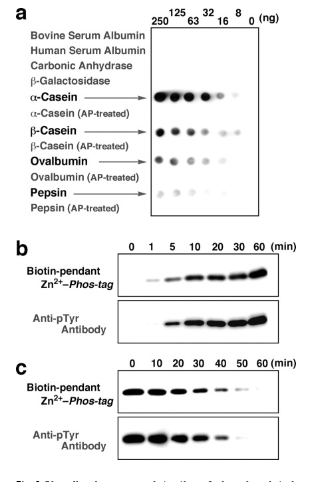
Figure 4: Chemiluminescence detection of phosphorylated proteins on PVDF membranes using biotin-pendant Zn2+-Phos-tag and HRP-SA.
When biotin is added to detection techniques, the signal can be amplified, increasing the sensitivity of phosphorylated protein identification. The Phos-tag BTL-104 can be easily immobilized onto streptavidin-coated surfaces thanks to the biotin tag, which makes affinity chromatography and pull-down experiments more effective. Using avidin/streptavidin-conjugated enzymes or fluorophores in Western blotting assays, the biotin tag makes it easier to detect phosphorylated proteins by generating a strong and targeted signal. Quantitative phosphoproteomic research may make use of phospho-tag Biotin BTL-104, which could advance our knowledge of cellular signaling cascades.
The wider use of Phos-tag technology in phosphorylation analysis is evidence of its value in expanding our understanding of cellular processes, even though the specifics of Phos-tag biotin BTL-104 can necessitate consulting the product documentation or the manufacturer. Phos-tag biotin BTL-104 has features and applications that researchers interested in protein phosphorylation and related fields should investigate, bearing in mind the potential benefits it may provide in their particular experimental contexts.













Comments