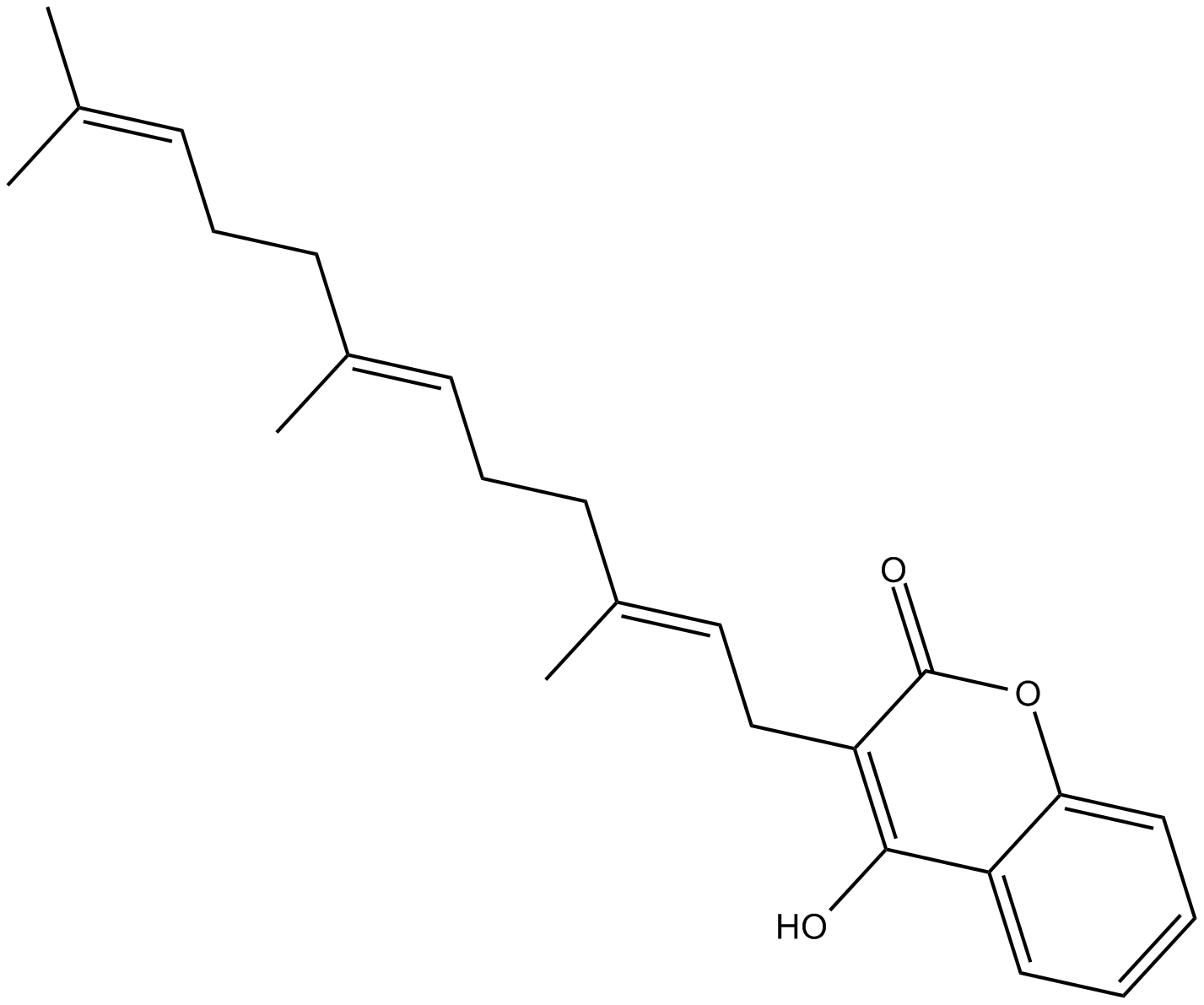
Ferulenol |
| Catalog No.GC12319 |
antimycobacterial activity and stimulates tubulin polymerization
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 6805-34-1
Sample solution is provided at 25 µL, 10mM.
Ferulenol is a prenylated 4-hydroxycoumarin derivative from Ferula communis var. genuine with haemorrhagic action [1]. It has been demonstrated that ferulenol exihibits potent antimycobacterial activity [2].
In vitro: Ferulenol stimulated tubulin polymerization in the absence of GTP, with a less extensive polymerization profile at 100 pM concentration. Ferulenol decreased radiolabeled colchicine bound by tubulin in a dose-dependent manner. Ferulenol altered the normal nuclear morphology of MCF-7 cells. Treatment with ferulenol (100 nM and 1 μM) for 24h induced a dose-dependent reduction of cell viability [3]
In vivo: In albino mice, the acute LD50s of ferulenol by single po or ip were 2,100 and 319 mg/kg bw, respectively. Three days after ferulenol administration, dosed animals showed hypoprothrombinemia with internal and external hemorrhages. Male mice were more sensitive to intoxication than females [4].
References:
[1] Lamnaouer D, Bodo B, Martin M T, et al. Ferulenol and ω-hydroxyferulenol, toxic coumarins from Ferula communis var. genuina[J]. Phytochemistry, 1987, 26(6): 1613-1615.
[2] E. Mamoci, I. Cavoski, V. Simone, et al. Chemical composition and in vitro activity of plant extracts from Ferula communis and Dittrichia viscosa against postharvest fungi. Molecules 16(3), 2609-2625 (2011).
[3] Bocca C, Gabriel L, Bozzo F, et al. Microtubule-interacting activity and cytotoxicity of the prenylated coumarin ferulenol[J]. Planta medica, 2002, 68(12): 1135-1137.
[4] Fraigui O, Lamnaouer D, Faouzi M Y. Acute toxicity of ferulenol, a 4-hydroxycoumarin isolated from Ferula communis L[J]. Veterinary and human toxicology, 2002, 44(1): 5-7.
Average Rating: 5 (Based on Reviews and 29 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















