Sofiniclin (Synonyms: ABT 894) |
| Catalog No.GC37662 |
Sofiniclin (ABT 894), an agonist of nicotinic acetylcholine receptor (nAChR), is used as a potential non-stimulant research for attention-deficit/hyperactivity disorder (ADHD).
Products are for research use only. Not for human use. We do not sell to patients.
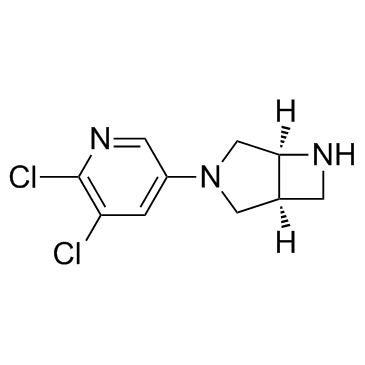
Cas No.: 799279-80-4
Sample solution is provided at 25 µL, 10mM.
Sofiniclin (ABT 894) is an agonist of nicotinic acetylcholine receptor (nAChR), used as a potential non-stimulant treatment for attention-deficit/hyperactivity disorder (ADHD).
Sofiniclin is more potent than ABT-089 at both receptor subtypes, with Ki values of 1.9 nM for 125I-α-conotoxinMII binding and of 1.3 nM for 125I-epibatidine binding[1].
Sofiniclin (0.001 to 0.10 mg/kg, p.o.) produces significant reductions in LIDs compared to vehicle monkey[1]. Sofiniclin (0.1 mg/kg) does not decrease LIDs in monkeys with severe nigrostriatal damage[2].
[1]. Zhang D, et al. ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson's disease. Mov Disord. 2014 Apr;29(4):508-17. [2]. Zhang D, ET AL. α7 nicotinic receptor agonists reduce levodopa-induced dyskinesias with severe nigrostriatal damage. Mov Disord. 2015 Dec;30(14):1901-11.
Average Rating: 5 (Based on Reviews and 40 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















