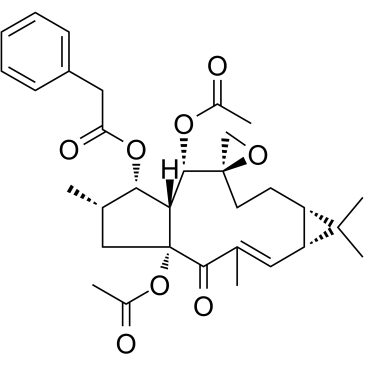
Euphorbiasteroid |
| Catalog No.GC38422 |
A tricyclic diterpene
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 28649-59-4
Sample solution is provided at 25 µL, 10mM.
Euphorbiasteroid is a tricyclic diperpene of Euphorbia lathyris L., inhibits tyrosinase, and increases the phosphorylation of AMPK, with anti-cancer, anti-virus, anti-obesity and multidrug resistance-modulating effect[1].
[1]. Park SJ, et al. Euphorbiasteroid, a component of Euphorbia lathyris L., inhibits adipogenesis of 3T3-L1 cells via activation of AMP-activated protein kinase. Cell Biochem Funct. 2015 Jun;33(4):220-5.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















