Reactive O2/N2 Pathways
Products for Reactive O2/N2 Pathways
- Cat.No. Product Name Information
-
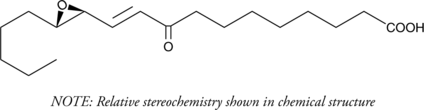
GC41213
(±)10-HDHA
(±)10-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
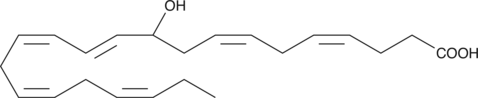
GC41214
(±)11-HDHA
(±)11-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
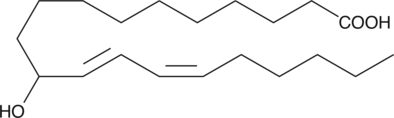
GC40428
(±)11-HEDE
(±)11-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.
-
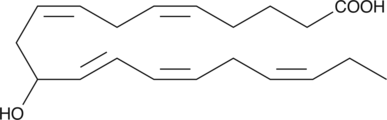
GC40387
(±)11-HEPE
(±)11-HEPE is produced by non-enzymatic oxidation of eicosapentaenoic acid.
-
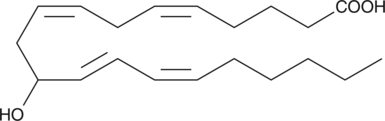
GC40467
(±)11-HETE
(±)11-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
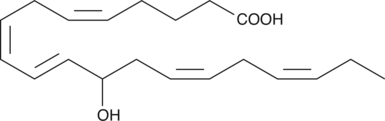
GC40359
(±)12-HEPE
(±)12-HEPE is produced by non-enzymatic oxidation of EPA.
-
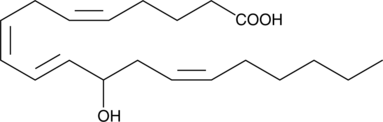
GC40429
(±)12-HETE
(±)12-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
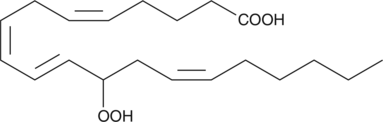
GC41115
(±)12-HpETE
(±)12-HpETE is one of the six monohydroperoxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid and consists of an equal mixture of the R and S isomers.
-
GC41192
(±)13-HDHA
(±)13-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
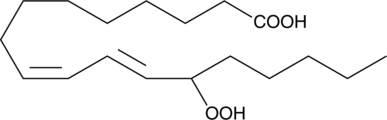
GC40355
(±)13-HpODE
(±)13-HpODE is a racemic mixture of hydroperoxides derived non-enzymatically from linoleic acid through the action of reactive oxygen species.
-
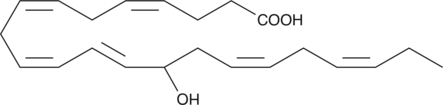
GC41193
(±)14-HDHA
(±)14-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
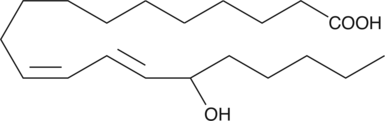
GC40420
(±)15-HEDE
(±)15-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.
-
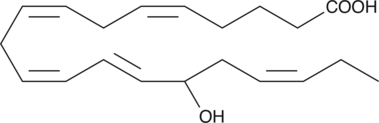
GC40361
(±)15-HEPE
(±)15-HEPE is produced by non-enzymatic oxidation of EPA.
-
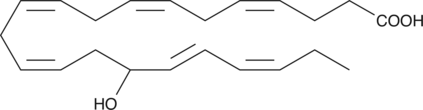
GC41196
(±)16-HDHA
(±)16-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
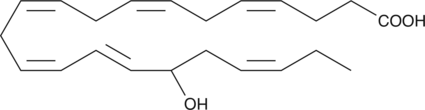
GC41197
(±)17-HDHA
(±)17-HDHA is an autoxidation product of docosahexaenoic acid in vitro.
-
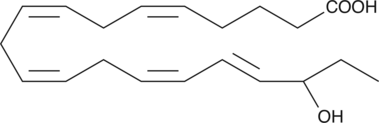
GC40362
(±)18-HEPE
(±)18-HEPE is produced by non-enzymatic oxidation of EPA.
-
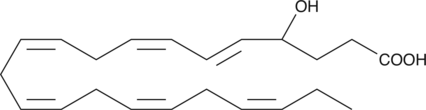
GC41202
(±)4-HDHA
(±)4-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
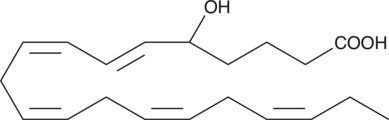
GC40364
(±)5-HEPE
(±)5-HEPE is produced by non-enzymatic oxidation of EPA.
-
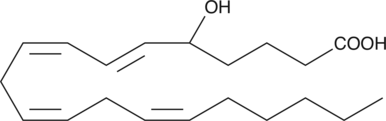
GC40439
(±)5-HETE
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
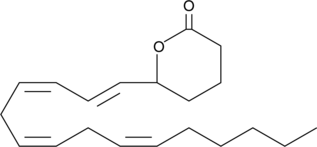
GC40828
(±)5-HETE lactone
(±)5-HETE lactone is a cyclic ester formed by acid-catalyzed nucleophilic addition of the C-5 hydroxyl to the C-1 carboxyl of (±)5-HETE.
-
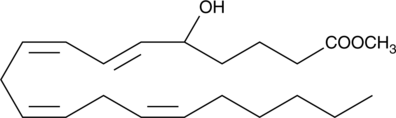
GC40837
(±)5-HETE methyl ester
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
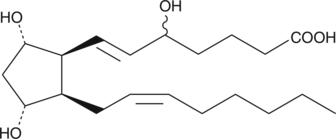
GC40576
(±)5-iPF2α-VI
Isoprostanes are prostaglandin (PG)-like products of free-radical induced lipid peroxidation.
-
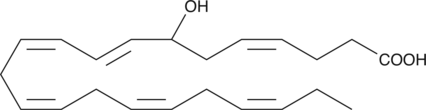
GC41204
(±)7-HDHA
(±)7-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
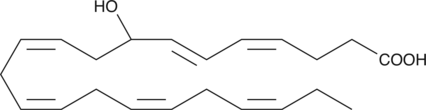
GC41205
(±)8-HDHA
(±)8-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
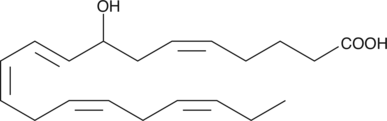
GC40366
(±)8-HEPE
(±)8-HEPE is produced by non-enzymatic oxidation of EPA.
-
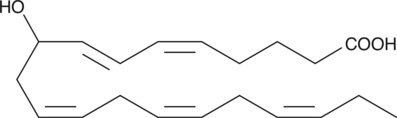
GC40367
(±)9-HEPE
(±)9-HEPE is produced by non-enzymatic oxidation of EPA.
-
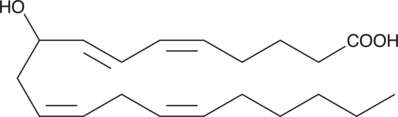
GC40443
(±)9-HETE
(±)9-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
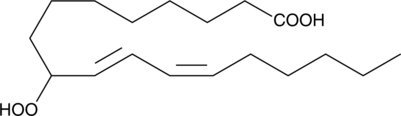
GC40356
(±)9-HpODE
(±)9-HpODE is a racemic mixture of the fatty acid hydroperoxide product (9(S)-HpODE) formed from lipoxygenase action on linoleic acid.
-
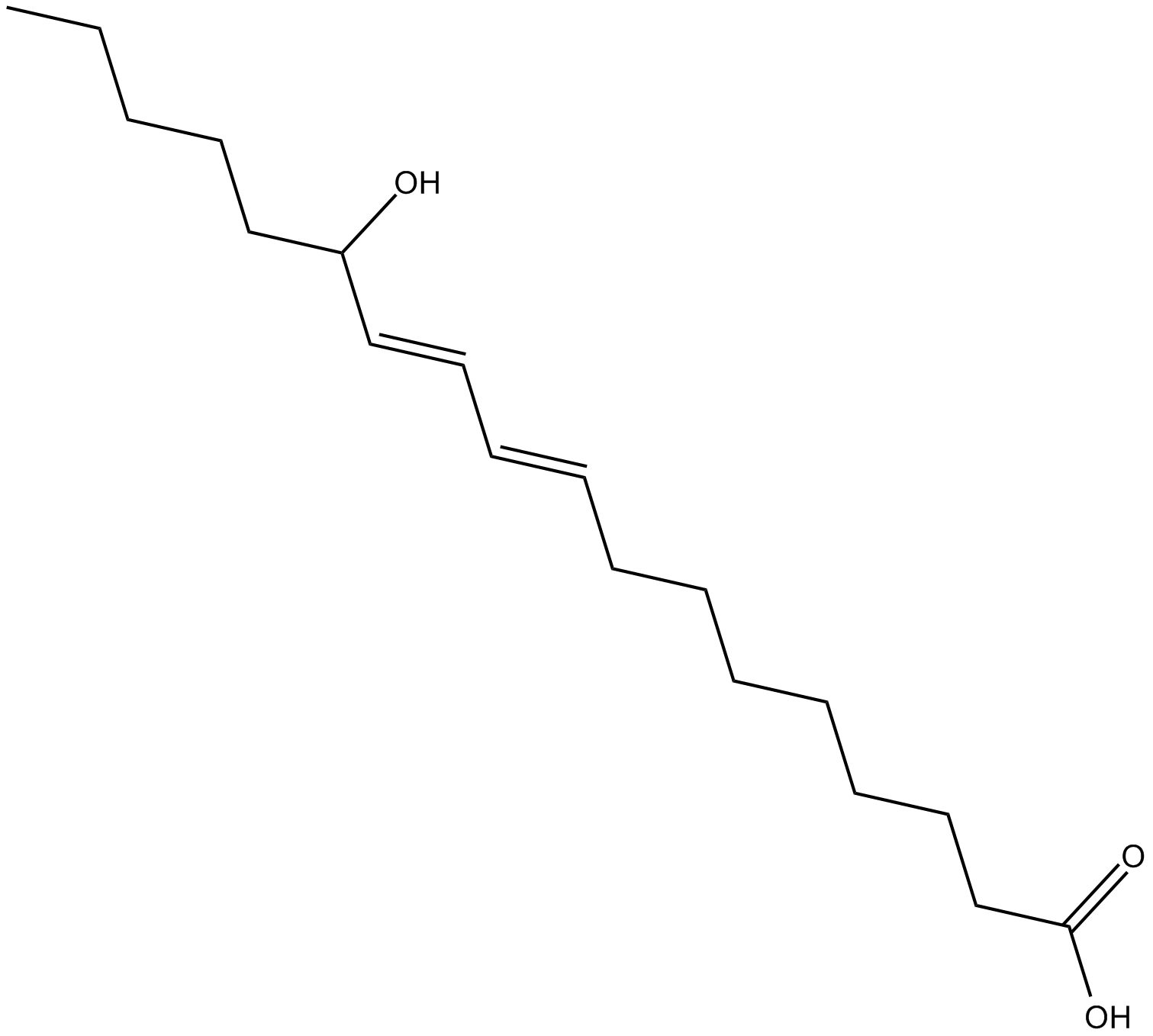
GC19461
(±)13-HODE
(±)13-HODE is one of the two racemic monohydroxy fatty acids resulting from the non-enzymatic oxidation of linoleic acid.
-
GC42023
1-Palmitoyl-2-Arachidonoyl-sn-glycero-3-PC
1-Palmitoyl-2-arachidonoyl-sn-glycero-3-PC (PAPC) is a phospholipid containing palmitic acid (16:0) and arachidonic acid (20:4) at the sn-1 and sn-2 positions, respectively, that is found in biological membranes.
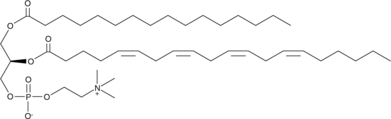
-
GC42042
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PE
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PE is a phospholipid that contains stearic acid at the sn-1 position and 15(S)-HpETE at the sn-2 position.
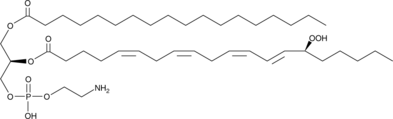
-
GC41868
10-Nitrooleate
10-Nitrooleate (CXA-10), a nitro fatty acid, has potential effects in disease states in which oxidative stress, inflammation, fibrosis, and/or direct tissue toxicity play significant roles.
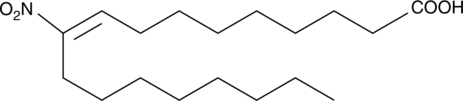
-
GC40368
11(R)-HEPE
11(R)-HEPE is produced by the oxidation of EPA by 11(R)-LO.
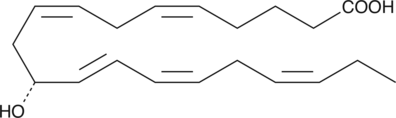
-
GC40370
12(R)-HEPE
12(R)-HEPE is a monohydroxy fatty acid synthesized from EPA by the eggs of the sea urchin, S.
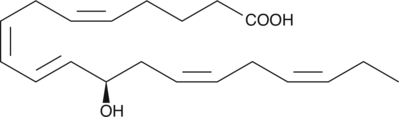
-
GC46420
13(S)-HODE-d4
An internal standard for the quantification of 13-HODE
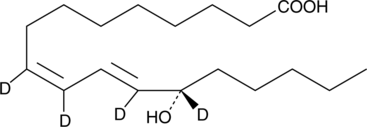
-
GC40426
15(R)-HEDE
15(R)-HEDE is isolated by the chromatographic resolution of (±)15-HEDE.
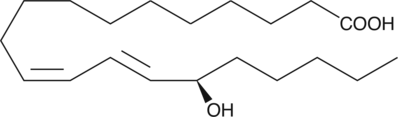
-
GC46442
15(S)-HETE-d8
An internal standard for the quantification of 15-HETE
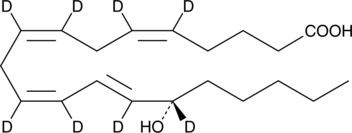
-
GC41928
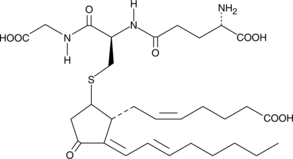
15-deoxy-δ12,14-Prostaglandin J2 Glutathione
15-deoxy-δ12,14-Prostaglandin J2 Glutathione (15-deoxy-δ12,14-PGJ2 Glutathione) is a non-enzymatic adduct formed from 15-deoxy-δ12,14-PGJ2 and glutathione.
-
GC40453
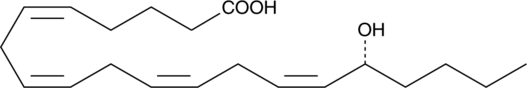
16(R)-HETE
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites.
-
GC41207
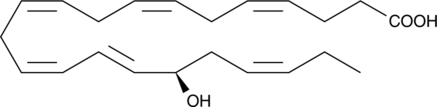
17(R)-HDHA
Resolvins are a group of polyhydroxylated metabolites of docosahexaenoic acid (DHA) found in the inflammatory exudates of aspirin-treated experimental animals.
-
GC41210
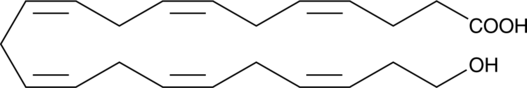
22-HDHA
22-HDHA is an oxidation product of docosahexaenoic acid.
-
GC42400
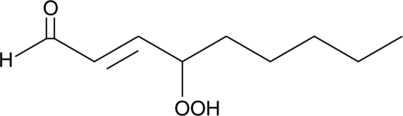
4-hydroperoxy 2-Nonenal
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids, such as linoleic acid and arachidonic acid, that is widely used as a marker of oxidative stress.
-
GC40778
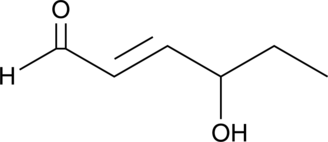
4-hydroxy Hexenal
4-hydroxy Hexenal is a lipid peroxidation product derived from oxidized ω-3 fatty acids such as DHA.
-
GC46656
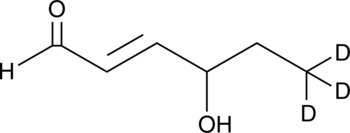
4-hydroxy Hexenal-d3
An internal standard for the quantification of 4-hydroxy hexenal
-
GC42411
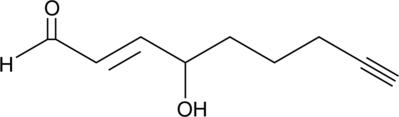
4-hydroxy Nonenal Alkyne
4-hydroxy Nonenal (4-HNE) is a major aldehyde produced during the lipid peroxidation of ω-6 polyunsaturated fatty acids, such as arachidonic acid and linoleic acid.
-
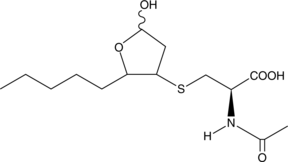
GC42413
4-hydroxy Nonenal Mercapturic Acid
Peroxidation of common ω-6 polyunsaturated fatty acids (PUFAs) such as linoleic acid, DGLA, and arachidonic acid can give rise to 4-HNE.
-
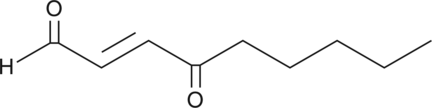
GC42464
4-oxo-2-Nonenal
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids such as arachidonic acid and linoleic acid.
-
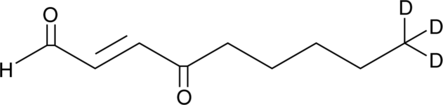
GC46674
4-oxo-2-Nonenal-d3
An internal standard for the quantification of 4oxo-2nonenal
-
GC40053
5α,6α-epoxy Cholestanol
An oxysterol and a metabolite of cholesterol produced by oxidation

-
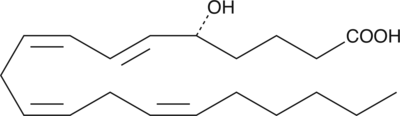
GC40459
5(R)-HETE
5(R)-HETE is a rare lipoxygenase product of arachidonic acid.
-
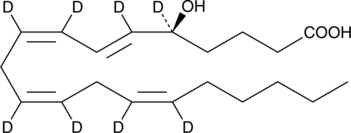
GC46679
5(S)-HETE-d8
An internal standard for the quantification of 5-HETE
-
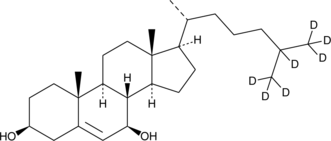
GC40201
7β-hydroxy Cholesterol-d7
7β-hydroxy Cholesterol-d7 is intended for use as an internal standard for the quantification of 7β-hydroxy cholesterol by GC- or LC-MS.
-
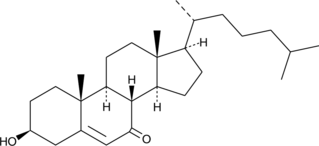
GC40572
7-keto Cholesterol
A bioactive sterol and a major oxysterol component of oxidized LDL
-
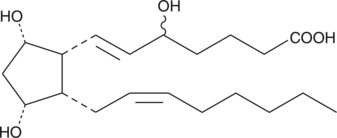
GC40587
8,12-iso-iPF2α-VI
8,12-iso-iPF2α-VI is an isoprostane produced by non-enzymatic, free radical-induced peroxidative damage to membrane lipids.
-
GC41138
8-iso Prostaglandin A1
8-iso Prostaglandin A1 (8-iso PGA1) is an isoprostane and a member in a large family of prostanoids of non-cyclooxygenase origin.
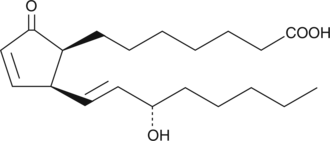
-
GC18781
8-iso Prostaglandin A2
Isoprostanes are prostaglandin (PG)-like compounds produced in vivo by free radical-catalyzed peroxidation of arachidonoyl-containing lipids.
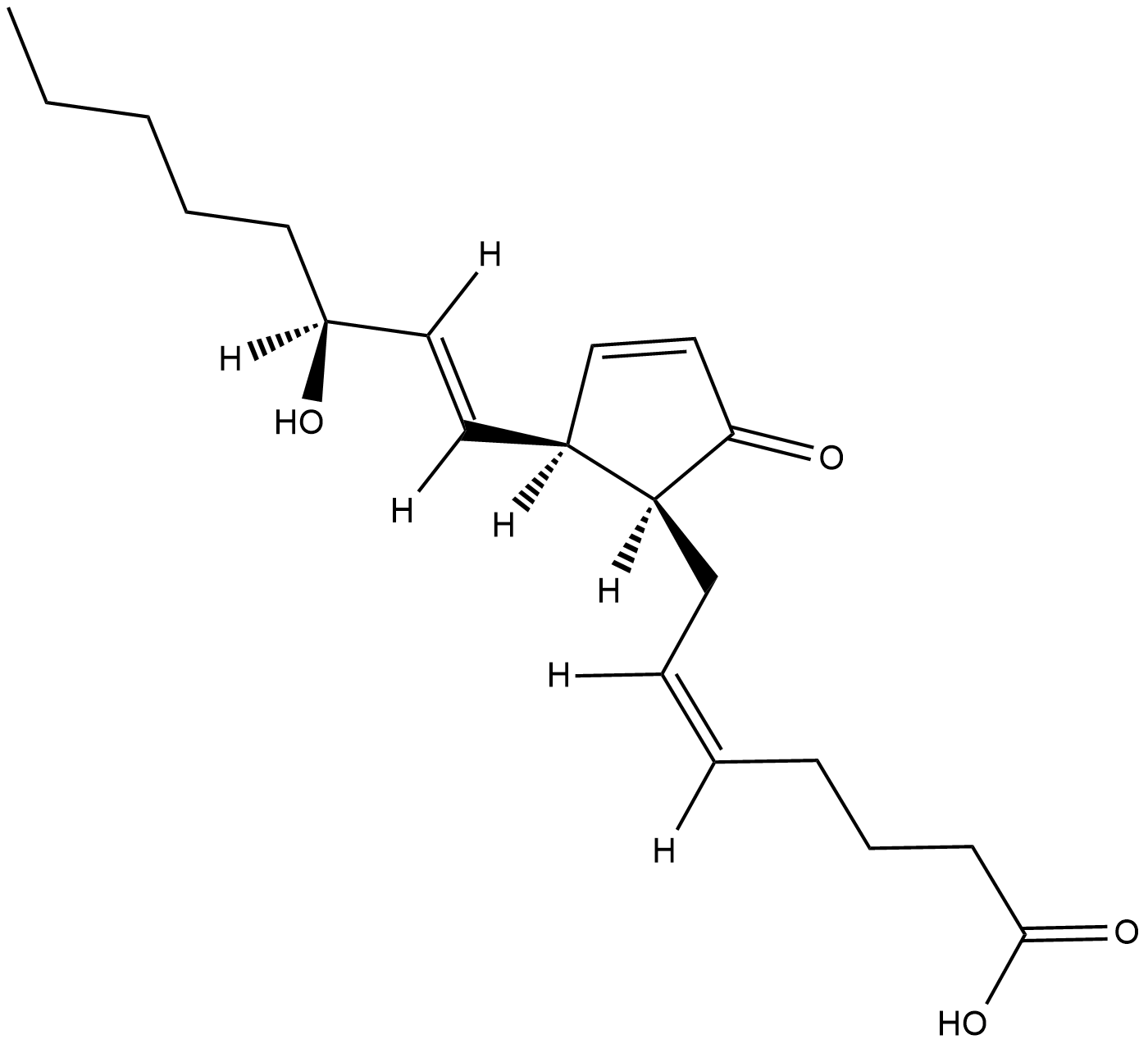
-
GC18737
8-iso Prostaglandin E1
Isoprostanes are a family of prostanoid molecules of non-cyclooxygenase origin.
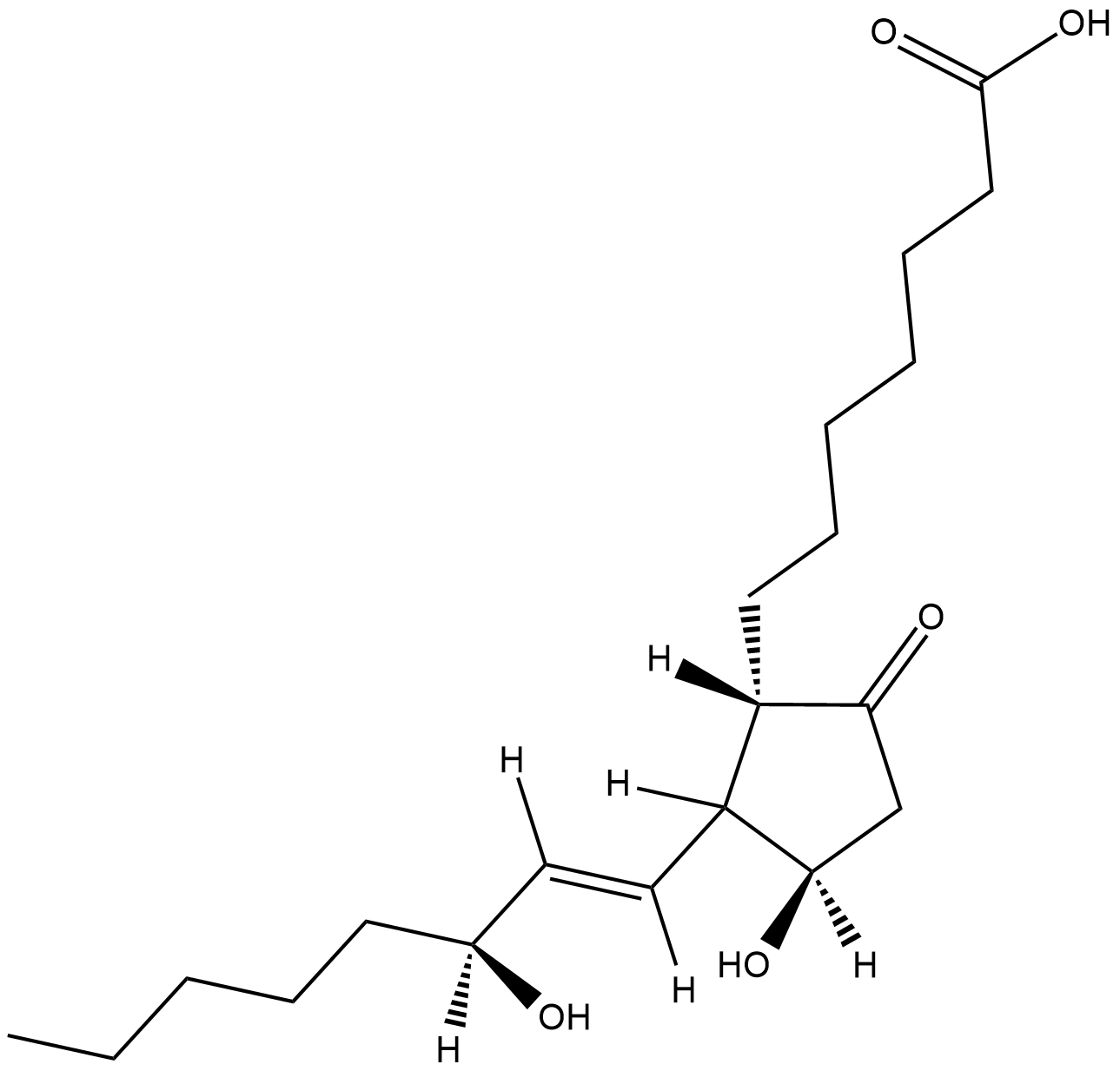
-
GC41425
8-iso Prostaglandin E2
8-iso PGE2 is one of several isoprostanes produced from arachidonic acid during lipid peroxidation.
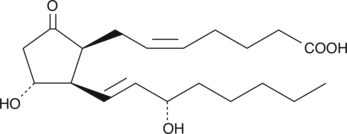
-
GC42629
8-iso Prostaglandin E2 isopropyl ester
8-iso PGE2 isopropyl ester is a more lipophilic form of the free acid, 8-iso PGE2.
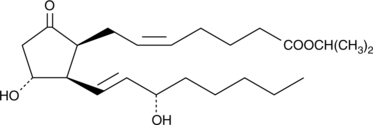
-
GC41437
8-iso Prostaglandin F1α
8-iso PGF1α is an isoprostane that was first identified in human semen.
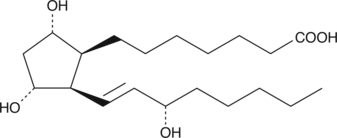
-
GC41438
8-iso Prostaglandin F1β
8-iso PGF1β is a potential autoxidation product of DGLA.
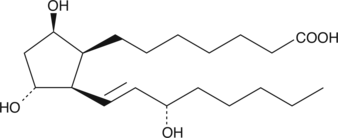
-
GC46747
8-iso Prostaglandin F2α-d4
An internal standard for the quantification of 8iso prostaglandin F2α
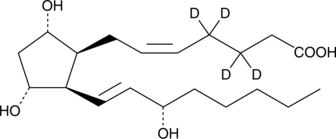
-
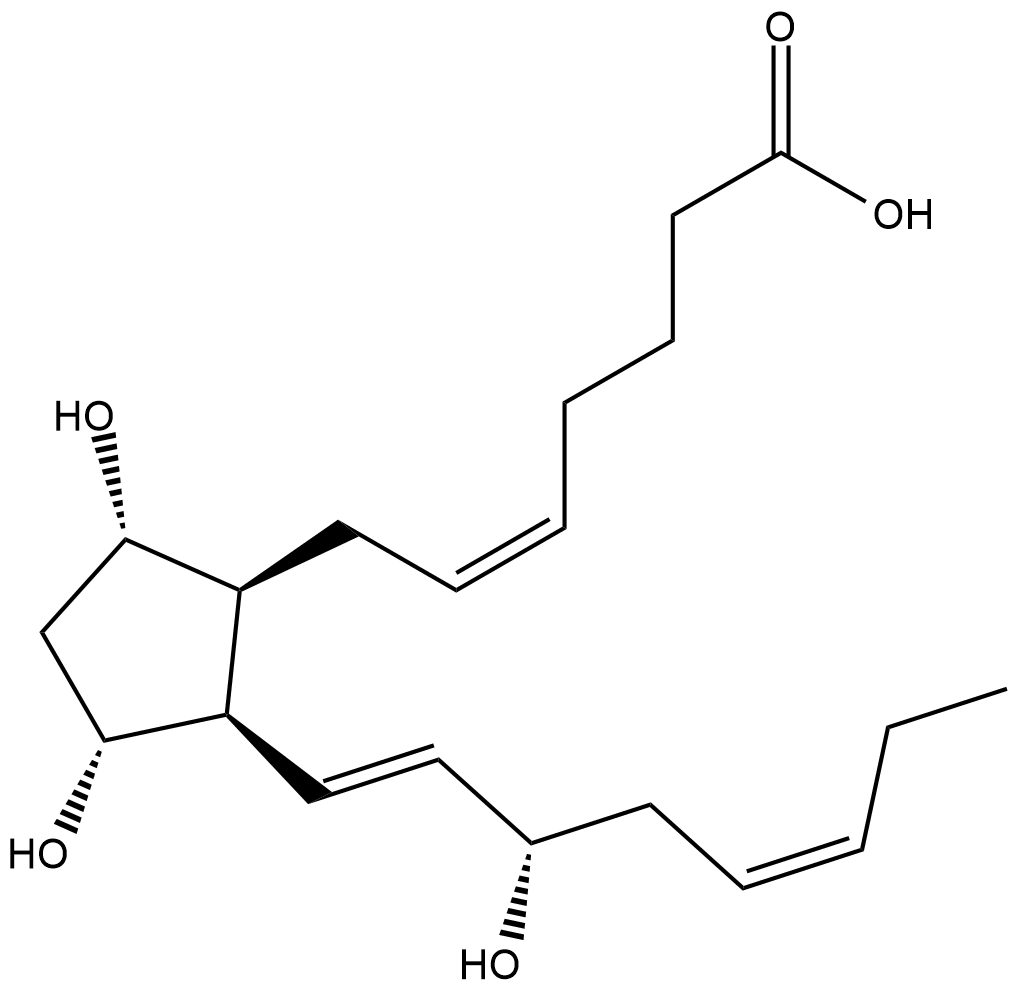
GC18829
8-iso Prostaglandin F3α
8-iso PGF3α is an isoprostane produced from the free-radical peroxidation of EPA.
-
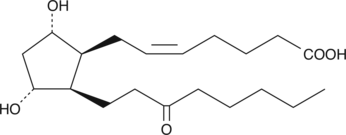
GC40588
8-iso-13,14-dihydro-15-keto Prostaglandin F2α
8-iso-13,14-dihydro-15-keto Prostaglandin F2α (8-iso-13,14-dihydro-15-keto PGF2α) is a metabolite of the isoprostane, 8-isoprostane (8-iso PGF2α), in rabbits, monkeys and humans.
-
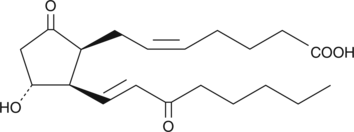
GC40608
8-iso-15-keto Prostaglandin E2
8-iso-15-keto Prostaglandin E2 (8-iso-15-keto PGE2) is an isoprostane, one member of a large family of biomarkers produced by the free radical peroxidative degradation of membrane lipids.
-
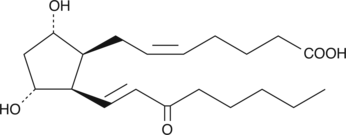
GC41426
8-iso-15-keto Prostaglandin F2α
8-iso-15-keto Prostaglandin F2α (8-iso-15-keto PGF2α) is a metabolite of the isoprostane 8-iso PGF2α in rabbits, monkeys, and humans.
-
GC41427
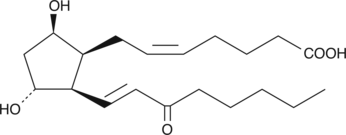
8-iso-15-keto Prostaglandin F2β
8-iso Prostaglandin F2β (8-iso PGF2β) is an isomer of PGF2α of non-enzymatic origin.
-
GC40332
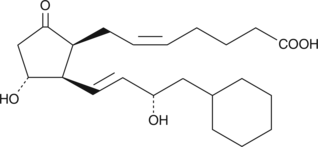
8-iso-16-cyclohexyl-tetranor Prostaglandin E2
8-iso Prostaglandin E2 (8-iso PGE2) is one of several isoprostanes produced from polyunsaturated fatty acids during lipid peroxidation.
-
GC46748
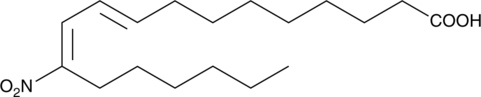
9(E),11(E)-12-nitro Conjugated Linoleic Acid
A nitrated fatty acid
-
GC46749
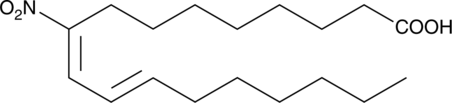
9(E),11(E)-9-nitro Conjugated Linoleic Acid
A nitrated fatty acid
-
GC40250
9(S)-HODE-d4 MaxSpec® Standard
9(S)-HODE-d4 is intended for use as an internal standard for the quantification of 9(S)-HODE by GC- or LC-mass spectrometry.
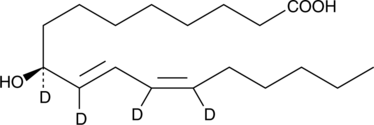
-
GC42649
9-Nitrooleate
Nitrated unsaturated fatty acids, such as 10- and 12-nitrolinoleate, cholesteryl nitrolinoleate, and nitrohydroxylinoleate, represent a new class of endogenous lipid-derived signalling molecules.
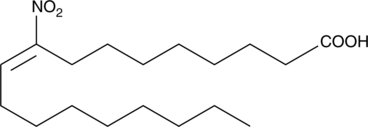
-
GC46761
9-Oxononanoic Acid
An oxidized fatty acid
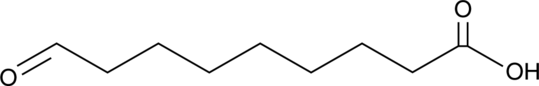
-
GC42658
A1-Phytoprostane-I
A1-Phytoprostane-I is a cyclopentenone isoprostane produced by the action of reactive oxygen species on α-linolenic acid in plants.
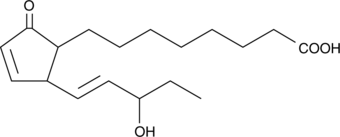
-
GC40545
cis-Parinaric Acid
cis-Parinaric acid is a naturally occurring polyunsaturated fatty acid containing an unusual conjugated (Z,E,E,Z) tetraene.

-
GC52470
Eicosanoids Metabolites MaxSpec® Discovery Mixture
A quantitative analytical standard mixture guaranteed to meet MaxSpec® identity, purity, stability, and concentration specifications

-
GC18986
ent-8-iso Prostaglandin F2α
Isoprostanes are produced by the non-enzymatic, free radical peroxidation of arachidonic acid.
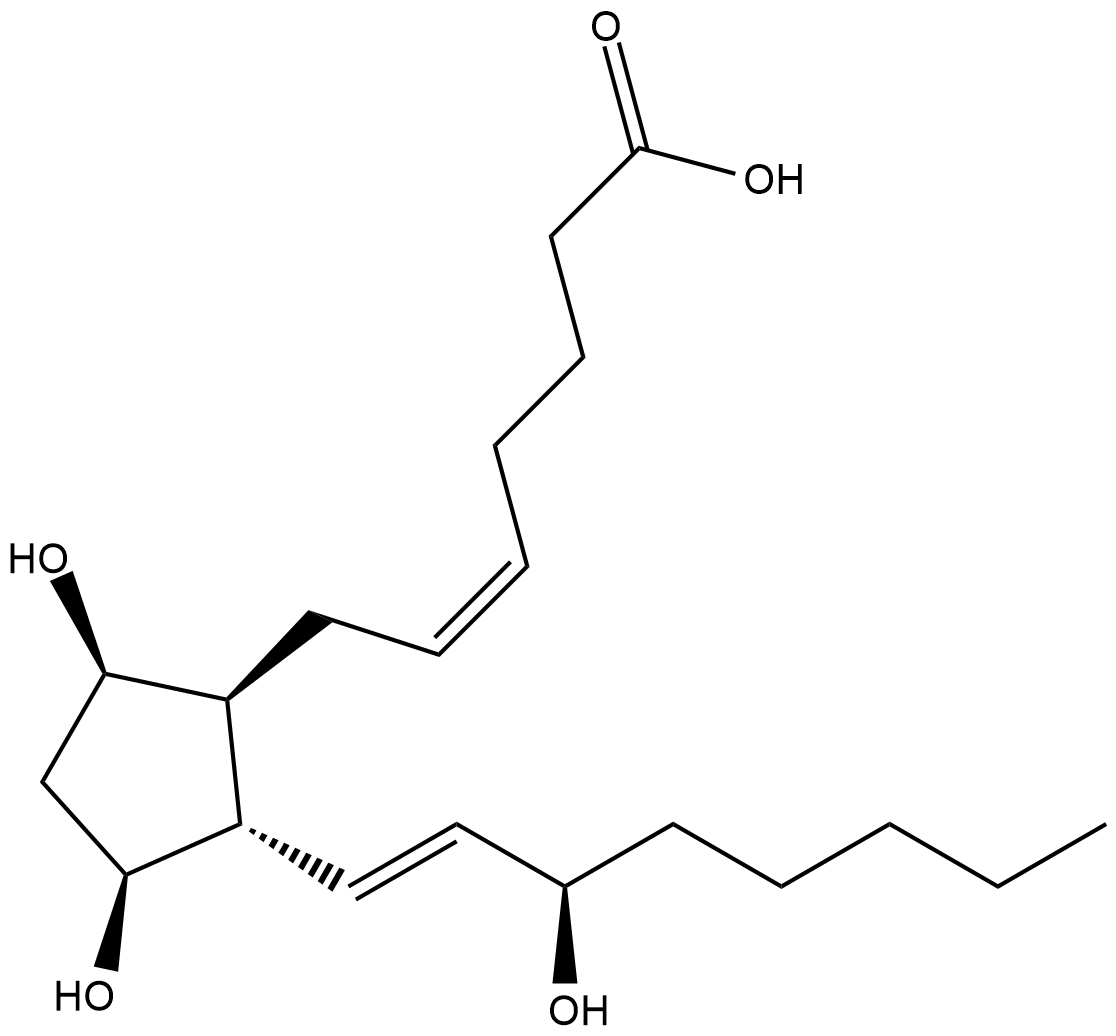
-
GC40590
ent-8-iso-15(S)-Prostaglandin F2α
Isoprostanes are produced by the non-enzymatic, free radical peroxidation of phospholipid-esterified arachidonic acid.
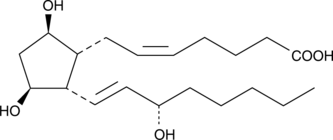
-
GC43997
KDdiA-PC
Oxidized low-density lipoprotein (oxLDL) particles contain low molecular weight species which are cytotoxic and pro-atherogenic.
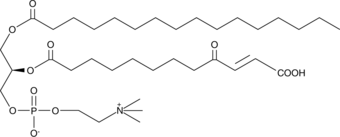
-
GC44011
KOdiA-PC
Oxidized low-density lipoprotien (oxLDL) particles contain low molecular weight species which are cytotoxic and pro-atherogenic.
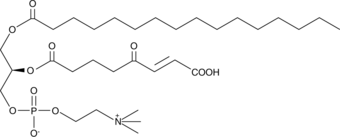
-
GC20172
N-Isopropylphthalimide

-
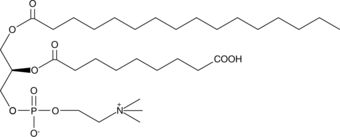
GC44573
PAz-PC
Oxidized low-density lipoprotein (oxLDL) particles contain low molecular weight species which are cytotoxic and pro-atherogenic.
-
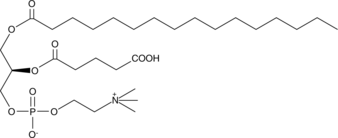
GC44616
PGPC
PGPC is an oxidized phospholipid that can be formed under conditions of oxidative stress.
-
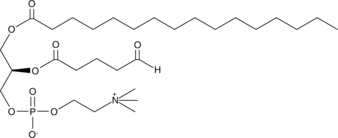
GC44669
POV-PC
Oxidized low-density lipoprotien (oxLDL) particles contain low molecular weight species which are cytotoxic and pro-atherogenic.
-
GC19446
trans-4,5-epoxy-2(E)-Decenal
Polyunsaturated fatty acids such as arachidonate and linoeate, while essential to health maintenance, are subject to random peroxidation by ambient oxygen, resulting in fragmented and reactive decomposition products.

-
GC45071
trans-EKODE-(E)-Ib
During oxidative stress, the abundant unsaturated fatty acid linoleic acid undergoes lipid peroxidation to produce α,β-unsaturated epoxy-keto-octadecenoic acids (EKODEs).