Mitomycin C (Synonyms: Ametycine) |
| Catalog No.GC12353 |
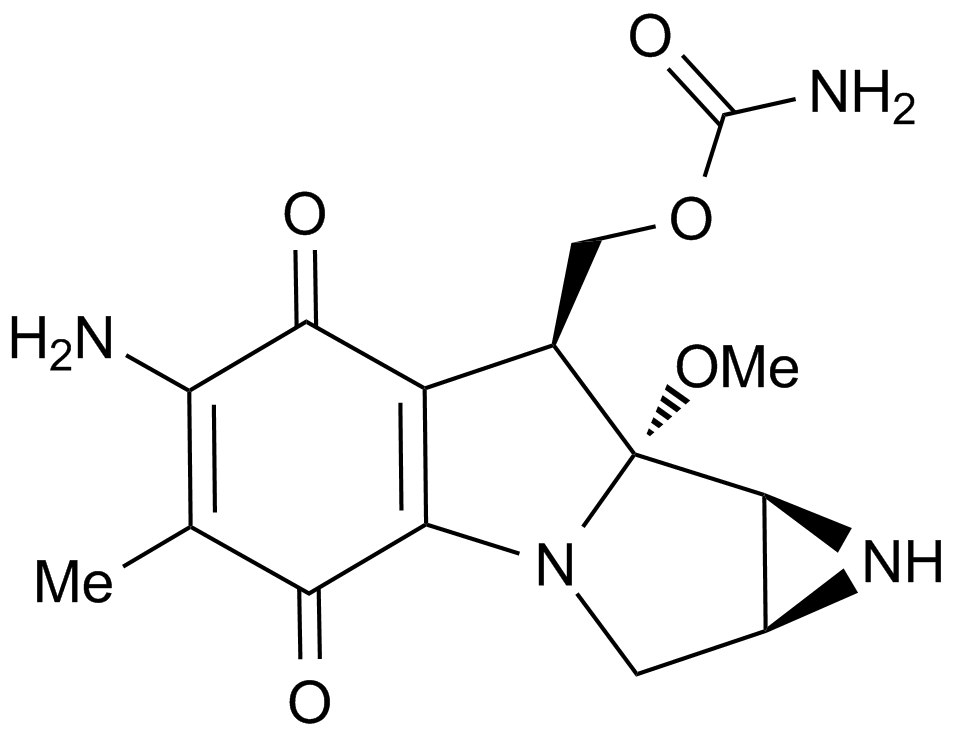
Mitomycin C is an antibiotic isolated from Streptomyces Caespitosus or Streptomyces Lavendulae. Mitomycin C inhibits DNA synthesis by forming covalent mitomycin C-DNA adducts with DNA, with an EC50 value of 0.14 μM in PC3 cells.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1950/7/7
Sample solution is provided at 25 µL, 10mM.
Mitomycin C is an antibiotic isolated from Streptomyces Caespitosus or Streptomyces Lavendulae. Mitomycin C inhibits DNA synthesis by forming covalent mitomycin C-DNA adducts with DNA, with an EC50 value of 0.14 μM in PC3 cells.
Mitomycin C can enhance the apoptosis effect induced by TRAIL on HCT116 (p53-/-) colon cancer cells, and can also make TRAIL-resistant colon cancer cells HT-29 sensitive to this cytokine. The IC50 of Mitomycin C on HT-29 cells is 40nM[1,2]. At the mechanistic level, Mitomycin C down-regulates cell survival-related proteins including Bcl2, Mcl-1, and Bcl-XL, and up-regulates the expression of pro-apoptotic proteins such as Bax, Bim, and TRAIL death receptors DR4 and DR5[1,2].
Mitomycin C has shown anti-tumor effects in animal experiments. In in vivo experiments, Mitomycin C significantly inhibited tumor growth and did not affect the body weight of mice under TRAIL treatment, indicating that the therapeutic combination of Mitomycin C and TRAIL was well tolerated in vivo and had antitumor activity[1].
Mitomycin C has demonstrated anti-tumor activity in preclinical and clinical studies and is widely used to treat various cancers. Mitomycin C is known to have synergistic effects with Capecitabine and Irinotecan. Studies have shown that in colorectal cancer, combination therapy of 5-FU or Raltiterxed with Mitomycin C is more effective than single agents[1].
In cell culture experiments, the recommended concentration of Mitomycin C is 0.2-20μg/mL.
References:
[1]. Cheng H, et al. Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: evidence for the role of c-Jun N-terminal kinase activation. Cell Cycle. 2012 Sep 1;11(17):3312-23.
[2]. Hodgkinson TJ, et al. Chemical synthesis and cytotoxicity of some azinomycin analogues devoid of the 1-azabicyclo[3.1.0]hexane subunit. Bioorg Med Chem Lett. 2000 Feb 7;10(3):239-41.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




