N-cis-hexadec-9Z-enoyl-L-Homoserine lactone (Synonyms: N-(2-oxotetrahydrofuran-3S-yl) Palmitoleyl Amide) |
| Catalog No.GC44336 |
Quorum sensing is a regulatory process used by bacteria for controlling gene expression in response to increasing cell density.
Products are for research use only. Not for human use. We do not sell to patients.
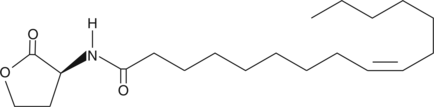
Cas No.: 479050-94-7
Sample solution is provided at 25 µL, 10mM.
Quorum sensing is a regulatory process used by bacteria for controlling gene expression in response to increasing cell density. This regulatory process manifests itself with a variety of phenotypes including biofilm formation and virulence factor production. Coordinated gene expression is achieved by the production, release, and detection of small diffusible signal molecules called autoinducers. The N-acylated homoserine lactones (AHLs) comprise one such class of autoinducers, each of which generally consists of a fatty acid coupled with homoserine lactone (HSL). AHLs vary in acyl group length (C4-C18), in the substitution of C3 (hydrogen, hydroxyl, or oxo group) and in the presence or absence of one or more carbon-carbon double bonds in the fatty acid chain. These differences confer signal specificity through the affinity of transcriptional regulators of the LuxR family. C16:1-δ9-(L)-HSL is a long-chain AHL that functions as a quorum sensing signaling molecule in strains of S. meliloti. Regulating bacterial quorum sensing signaling can be used to inhibit pathogenesis and thus, represents a new approach to antimicrobial therapy in the treatment of infectious diseases.
Reference:
[1]. González, J.E., and Keshavan, N.D. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 70(4), 859-875 (2006).
[2]. Gould, T.A., Herman, J., Krank, J., et al. Specificity of acyl-homoserine lactone syntheses examined by mass spectrometry. J. Bacteriol. 188(2), 773-783 (2006).
[3]. Penalver, C.G.N., Morin, D., Cantet, F., et al. Methylobacterium extorquens AM1 produces a novel type of acyl-homoserine lactone with a double unsaturated side chain under methylotrophic growth conditions. FEBS Lett. 580(2), 561-567 (2006).
[4]. Teplitski, M., Eberhard, A., Gronquist, M.R., et al. Chemical identification of N-acyl homoserine lactone quorum-sensing signals produced by Sinorhizobium meliloti strains in defined medium. Archives of Microbiology 180, 494-497 (2003).
[5]. Gao, M., Chen, H., Eberhard, A., et al. sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti. Journal of Bacteriology 187(23), 7931-7944 (2005).
[6]. Marketon, M.M., Glenn, S.A., Eberhard, A., et al. Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. Journal of Bacteriology 185(1), 325-331 (2003).
[7]. Marketon, M., Gronquist, M.R., Eberhard, A., et al. Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-Acyl homoserine lactones. Journal of Bacteriology 184(20), 5686-5695 (2002).
[8]. Cegelski, L., Marshall, G.R., Eldridge, G.R., et al. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6(1), 17-27 (2008).
Average Rating: 5 (Based on Reviews and 23 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















