Phentolamine-d4 (hydrochloride) |
| Catalog No.GC45999 |
An internal standard for the quantification of phentolamine
Products are for research use only. Not for human use. We do not sell to patients.
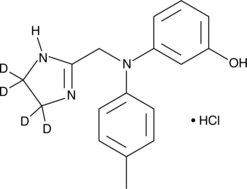
Cas No.: 1346599-65-2
Sample solution is provided at 25 µL, 10mM.
Phentolamine-d4 is intended for use as an internal standard for the quantification of phentolamine by GC- or LC-MS. Phentolamine is a reversible antagonist of α-adrenergic receptors, non-specifically binding all α1- and α2-adrenoceptors with nanomolar affinities.1,2,3,4 Formulations containing phentolamine have been used in the treatment of hypertensive emergencies, as well as chronic and emergent pain.
|1. Lomasney, J.W., Cotecchia, S., Lorenz, W., et al. Molecular cloning and expression of the cDNA for the α1A-adrenergic receptor. The gene for which is located on human chromosome 5. J. Biol. Chem. 266(10), 6365-6369 (1991).|2. Millan, M.J., Newman-Tancredi, A., Audinot, V., et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at α2-adrenergic receptors (AR)s, serotonin (5-HT)1A, 5-HT1B, 5-HT1D and dopamine D2 and D3 receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse 35(2), 79-95 (2000).|3. O'Rourke, M.F., Iversen, L.J., Lomasney, J.W., et al. Species orthologs of the Alpha-2A adrenergic receptor: The pharmacological properties of the bovine and rat receptors differ from the human and porcine receptors. J. Pharmacol. Exp. Ther. 271(2), 735-740 (1994).|4. Richelson, E., and Nelson, A. Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J. Pharmacol. Exp. Ther. 230(1), 94-102 (1984).
Average Rating: 5 (Based on Reviews and 15 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















