SB-649868 (Synonyms: GSK 649868) |
| Catalog No.GC19323 |
SB-649868 is a potent and selective orally active orexin (OX) 1 and OX2 receptor antagonist (pKi =9.4 and 9.5 at the OX1 and OX2 receptor, respectively).
Products are for research use only. Not for human use. We do not sell to patients.
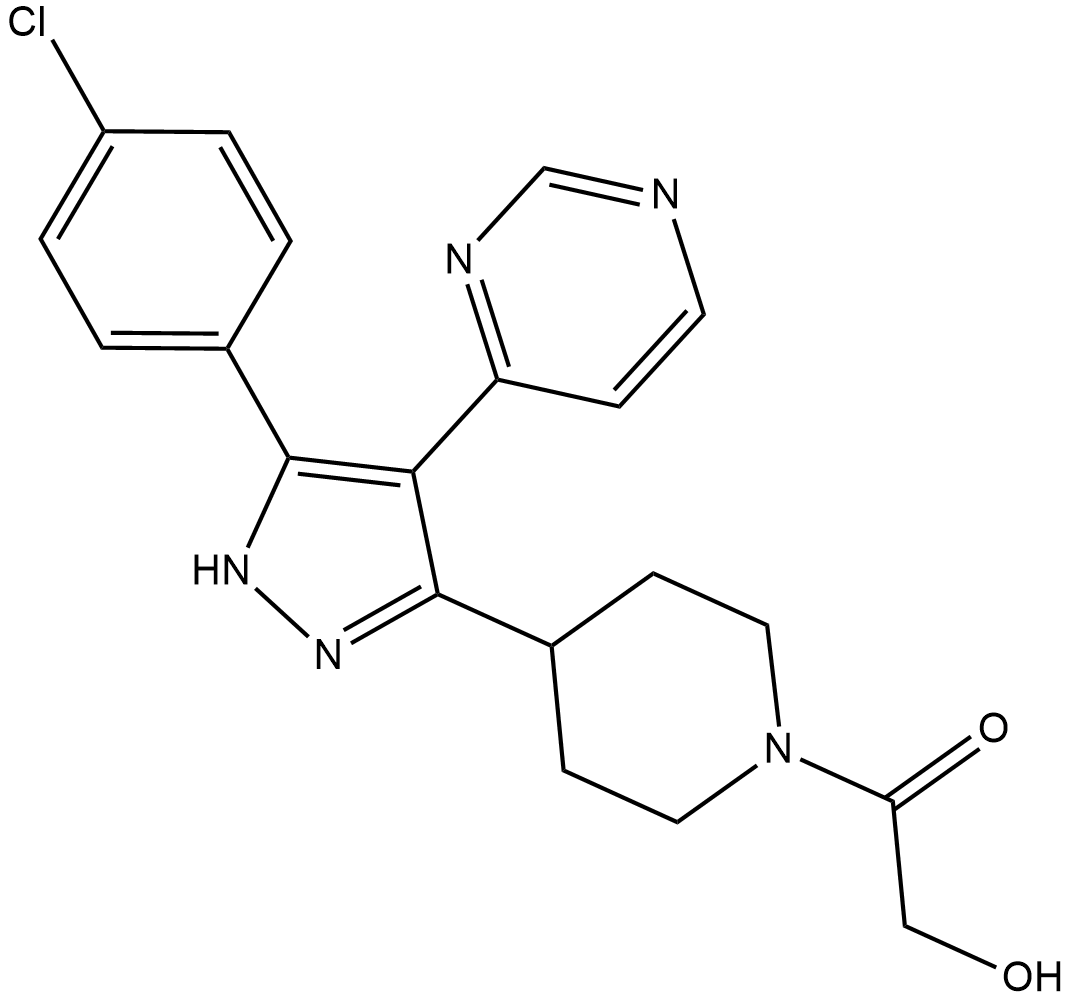
Cas No.: 380899-24-1
Sample solution is provided at 25 µL, 10mM.
SB-649868 is a potent and selective orally active orexin (OX) 1 and OX2 receptor antagonist (pKi =9.4 and 9.5 at the OX1 and OX2 receptor, respectively).
SB-649868 is identified as one the most in vitro potent dual OX1 and OX2 receptor antagonist known at that time (pKi=9.4 and 9.5 at the OX1 and OX2 receptor, respectively) [1]. SB-649868 antagonizes orexin-A-induced inositol 1 phosphate (IP1) accumulation with the following pKB value (OX1=9.67; OX2=9.64). SB-649868 displaces the [3H]ACT-078573 receptor binding with the following pKi values: OX1=9.27; OX2=8.91. Increasing concentrations of SB-649868 (0.3 nM-30 nM) induces a rightward shift of the orexin-A CRCs with a depression of the agonist efficacy suggesting a clear non-surmountable behavior. The calculated apparent pKb values are 9.67±0.03 and 9.64±0.07 for OX1 and OX2[2].
Pharmacokinetic studies in the male CD rat, performed at 1 mg/kg, iv and 3 mg/kg, po, demonstrate an excellent pharmacokinetic profile for a hypnotic agent featuring moderate clearance in plasma (Clp=24 mL/min/kg), short half-life of (<0.6 h) and a low volume of distribution (Vss=1.1 l/kg), coupled with excellent oral bioavailability (F=85%) and good exposure in plasma (Cmax=333 ng/mL). A brain to blood ratio (B/B) of 0.1:1 is observed 1 h after iv administration, a value in line with the expected partition between the two compartments based on the lower tissue binding observed in vitro in brain tissues (fraction unbound/brain=5.28%) with respect to plasma proteins (fraction unbound/plasma=1.34%). SB-649868, administered orally 3 h before OX-A injection at doses of 1, 3 and 10 mg/kg, causes a dose-dependent reduction of OX-A induced grooming as measured by total time spent grooming and number of grooming bouts (p <0.01 at 3 and 10 mg/kg po) [1]. From dissociation kinetic studies using [3H]ACT-078573, the calculated long half-life, (t1/2) supports the non-surmountability profile of SB-649868 (t1/2=35.91 min) at OX1 orexin receptor. The long or moderately long t1/2values for SB-649868 at OX2 orexin receptor (t1/2=8.09 min)[2].
References:
[1]. Di Fabio R, et al. Discovery process and pharmacological characterization of a novel dual orexin 1 and orexin 2receptor antagonist useful for treatment of sleep disorders. Bioorg Med Chem Lett. 2011 Sep 15;21(18):5562-7.
[2]. Faedo S, et al. Functional and binding kinetic studies make a distinction between OX1 and OX2 orexin receptorantagonists. Eur J Pharmacol. 2012 Oct 5;692(1-3):1-9.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















