Tasquinimod (Synonyms: ABR-215050) |
| Catalog No.GC14340 |
An orally-active anti-cancer compound
Products are for research use only. Not for human use. We do not sell to patients.
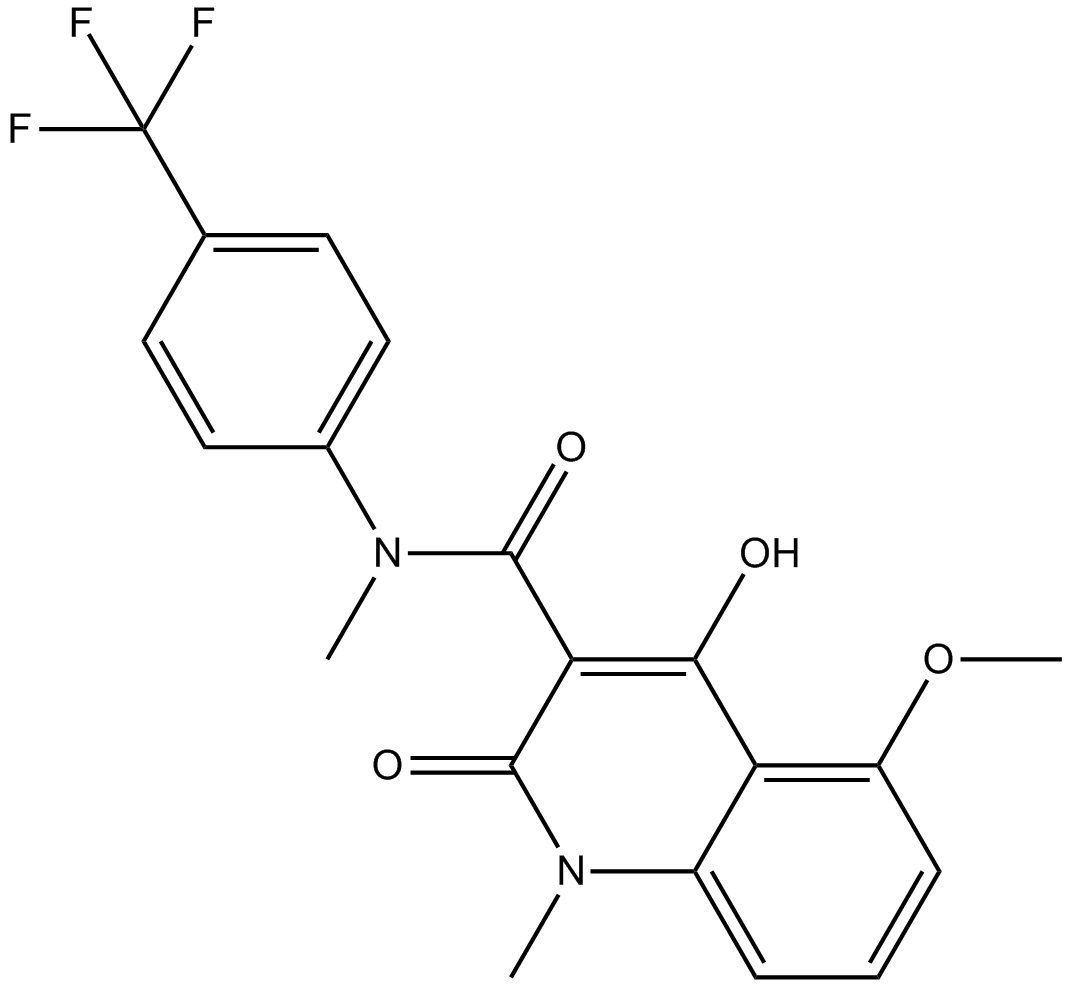
Cas No.: 254964-60-8
Sample solution is provided at 25 µL, 10mM.
Tasquinimod is an orally administered quinoline-3-carboxamide with potent antiangiogenic and antitumorigenic ability that has shown promise in the treatment of advanced prostate cancers [1].
Treatment with tasquinimod leads to a remarkable up-regulation in the expression of TSP-1 and down-regulation of VEGF and HIF-1α. The antiangiogenic activities of tasquinimod are therefore due to the dual inhibition of S100A9/TLR4 in MDSCs and the inhibition of HDAC4/N-CoR/HDACs deacetylation of HIF1-α in both endothelial and tumor cells, inhibiting hypoxia induced angiogenesis.
Human endothelial and prostate cancer cells in culture and human prostate cancer xenografts growing in castrated male nude mice were evaluated for their response to radiation alone and in combination with tasquinimod. Due to its potent reduction of the hypoxic response in endothelial cells, cancer cells, TAMs and MDSCs, tasquinimod inhibits tumor angiogenesis while sparing already formed vasculature. The data obtained in vivo and in vitro highlights a potent anticancer effect as a monotherapy in addition to greatly improving the response to combination therapies with docetaxel, androgen deprivation therapy or radiotherapy [1, 3].
At clinically relevant drug levels, tasquinimod significantly enhances anti-cancer efficacy of fractionated radiation with optimal timing for initiating daily tasquinimod treatment being after completion of the fractionated radiation. Phase I and II studies of tasquinimod have demonstrated tasquinimod to be well-tolerated and lead to significant improvements in progression-free survival from metastasis, by a period of 4.3 months, in patients with minimally symptomatic CRPC. The result highlights tasquinimod as an extremely promising and much needed therapeutic tool for use in CRPC [1, 2].
References:
[1]. Williamson SC, Hartley AE, Heer R. A review of tasquinimod in the treatment of advanced prostate cancer. Drug Design Development And Therapy, 2013, 7: 167-174.
[2]. Olsson A, Bjork A, Vallon-Christersson J, et al. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Molecular Cancer, 2010, 9: 107.
[3]. Dalrymple SL, Becker RE, Zhou HM, et al. Tasquinimod prevents the angiogenic rebound induced by fractionated radiation resulting in an enhanced therapeutic response of prostate cancer xenografts. Prostate, 2012, 72(6): 638-648.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















