U 18666A |
| Catalog No.GC15284 |
U18666A can inhibit 3β-hydroxysterol-Δ (24)-reductase (DHCR24) activity, thereby preventing cholesterol outflow from late endosomes and lysosomes.
Products are for research use only. Not for human use. We do not sell to patients.
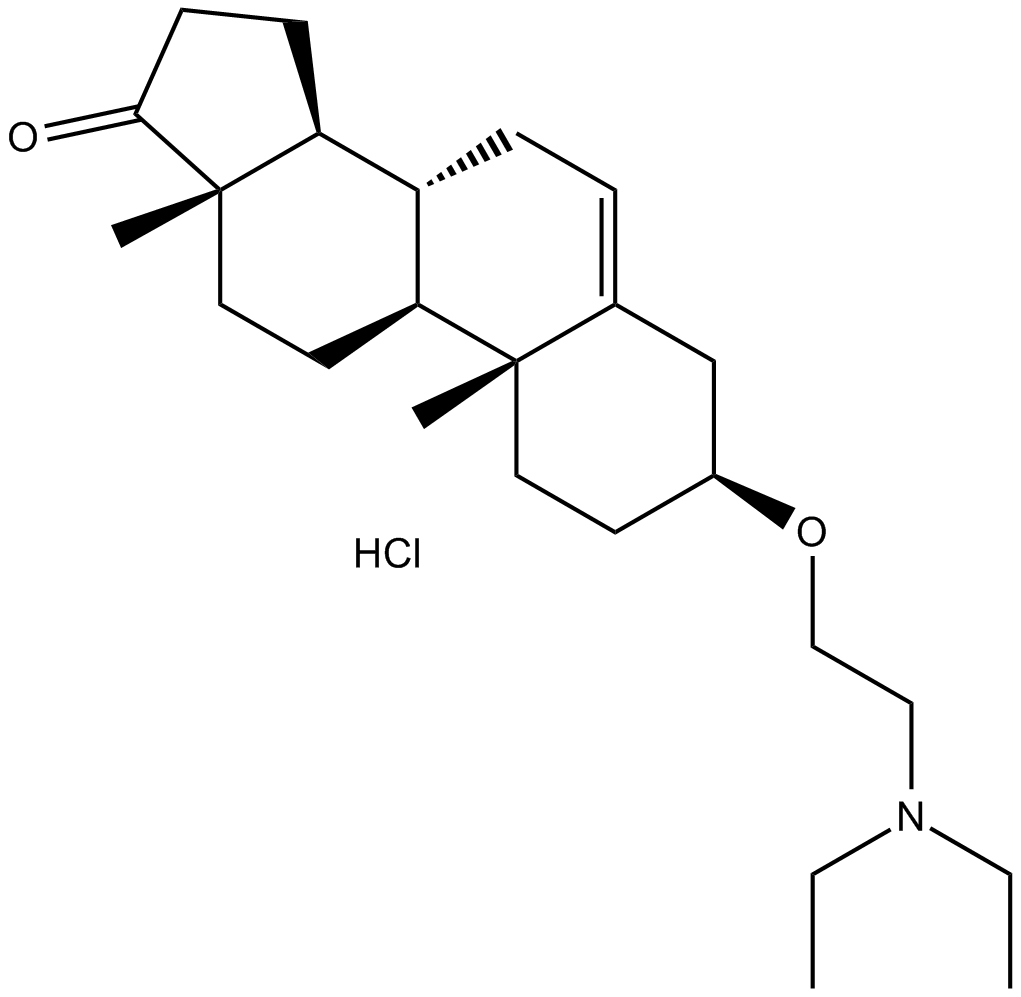
Cas No.: 3039-71-2
Sample solution is provided at 25 µL, 10mM.
U18666A is a small molecule with cellular permeability and amphiphilism that inhibits 3β-hydroxysterol-Δ (24)-reductase (DHCR24) activity [1], thereby preventing cholesterol outflow from late endosomes and lysosomes [2]. U18666A has been used to model three diseases: Niemann-Pick disease, epilepsy and cataracts [3].
U18666A(0.1μg/μl) can inhibit intracellular transport and biosynthesis of cholesterol in Chinese hamster cells (CHO) [4]. In addition, U18666A (1 μg/ml,24h) can prevent the transfer of CD63/Lamp-3 from late endosomes to Weibel-palade bodiesin Human Umbilical Vein Endothelial Cells (HUVEC) [5].
U18666A(10mg/kg) given to Sprague-Dawley rats every other day beginning at 1 day of age resulted in dense nuclear cataract [6]. The rats were injected weekly with U18666A (10 mg/kg, s.c.) starting on day 1 after birth, and the rats were in a state of spontaneous seizures starting at sixth week [7].
References:
[1] Quan X, Chen X, Sun D, Xu B, Zhao L, Shi X, Liu H, Gao B, Lu X. The mechanism of the effect of U18666a on blocking the activity of 3β-hydroxysterol Δ-24-reductase (DHCR24): molecular dynamics simulation study and free energy analysis. J Mol Model. 2016 Feb;22(2):46. doi: 10.1007/s00894-016-2907-. Epub 2016 Jan 27. PMID: 26815033.
[2] Cenedella RJ. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009 Jun;44(6):477-87. doi: 10.1007/s11745-009-3305-7.Epub 2009 May 14. PMID: 19440746.
[3] Koh CH, Cheung NS. Cellular mechanism of U18666A-mediated apoptosis in cultured murine cortical neurons: bridging Niemann-Pick disease type C and Alzheimer's disease. Cell Signal. 2006 Nov;18(11):1844-53. doi: 10.1016/j.cellsig.2006.04.006. Epub 2006 May 7. PMID: 16797161.
[4] Liscum L, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino) ethoxy] androst-5-en-17-one. J Biol Chem. 1989 Jul 15;264(20):11796-806. PMID: 274541.
[5] Kobayashi T, Vischer UM, Rosnoblet C, Lebrand C, Lindsay M, Parton RG, Kruithof EK, Gruenberg J. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell. 2000 May;11(5):1829-43. doi: 10.1091/mbc.11.5.1829. PMID: 10793155; PMCID: PMC14887.
[6] Alcala J, Cenedella RJ, Katar M. Limited proteolysis of MP26 in lens fiber plasma membranes of the U18666A-induced cataract in rats. Curr Eye Res. 1985 Sep;4(9):1001-5. doi: 10.3109/02713689509000008. PMID: 3905265.
[7] Bierkamper GG, Cenedella RJ. Induction of chronic epileptiform activity in the rat by an inhibitor of cholesterol synthesis, U18666A. Brain Res. 1978 Jul 14;150(2):343-51. doi: 10.1016/0006-8993(78)90285-8. PMID: 678974.
Average Rating: 5 (Based on Reviews and 28 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















