UPF-648 (Synonyms: (±)-DBCC) |
| Catalog No.GC30882 |
An inhibitor of kynurenine 3-monooxygenase
Products are for research use only. Not for human use. We do not sell to patients.
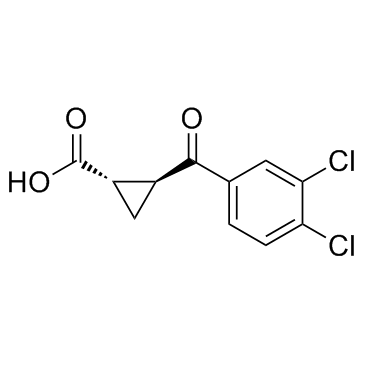
Cas No.: 213400-34-1
Sample solution is provided at 25 µL, 10mM.
UPF-648 is a potent kynurenine 3-monooxygenase (KMO) inhibitor; exhibits highly active at 1 uM (81 ± 10% KMO inhibition); ineffective at blocking KAT activity.IC50 value: 1 uM(81 ± 10 % inhibition) [1]Target: KMO inhibitorin vitro: BFF 122 inhibited KAT activity almost completely at both 1 and 0.1 mM. The effect was still remarkable at 0.01 mM (70 ± 1 % inhibition). At the same three concentrations, BFF 122 did not affect KMO activity significantly. In contrast, UPF 648 totally blocked KMO at 0.1 and 0.01 mM and was still highly active at 0.001 mM (81 ± 10 % inhibition), but the compound was essentially ineffective at blocking KAT activity [1]. UPF 648 binds close to the FAD cofactor and perturbs the local active-site structure, preventing productive binding of the substrate l-kynurenine. Functional assays and targeted mutagenesis reveal that the active-site architecture and UPF 648 binding are essentially identical in human KMO, validating the yeast KMO-UPF 648 structure as a template for structure-based drug design [3].in vivo: Applying an identical experimental design, separate rats were used to study the effect of KMO inhibition on the de novo synthesis of KP metabolites in the lesioned striatum. These animals were bilaterally injected with 0.1 mM UPF 648 and 3H-kynurenine in PBS. 0.1 mM UPF 648 significantly reduced the neosynthesis of 3-HK and QUIN in the lesioned striatum (by 77 % and 66%, respectively) and moderately (27%) but significantly increased the de novo formation of KYNA [1]. Administered to pregnant rats or mice on the last day of gestation, UPF 648 (50 mg/kg, i.p.) produced qualitatively similar changes (i.e., large increases in kynurenine and KYNA and reductions in 3-HK and QUIN) in the brain and liver of the offspring. Rat pups delivered by UPF 648-treated mothers and immediately exposed to neonatal asphyxia showed further enhanced brain KYNA levels [2]. UPF 648, has an IC50 of 20 nM and provides protection against intrastriatal QUIN injections in kynurenine aminotransferase (KAT II) deficient mice. UPF 648 treatment also shifts KP metabolism towards enhanced neuroprotective KYNA formation [3].
[1]. Amori L, et al. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem. 2009 Apr;109(2):316-25. [2]. Ceresoli-Borroni G, et al. Perinatal kynurenine 3-hydroxylase inhibition in rodents: pathophysiological implications. J Neurosci Res. 2007 Mar;85(4):845-54. [3]. Amaral M, et al. Structural basis of kynurenine 3-monooxygenase inhibition. Nature. 2013 Apr 18;496(7445):382-5.
Average Rating: 5 (Based on Reviews and 27 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















