Abacavir |
| Catalog No.GC13805 |
Products are for research use only. Not for human use. We do not sell to patients.
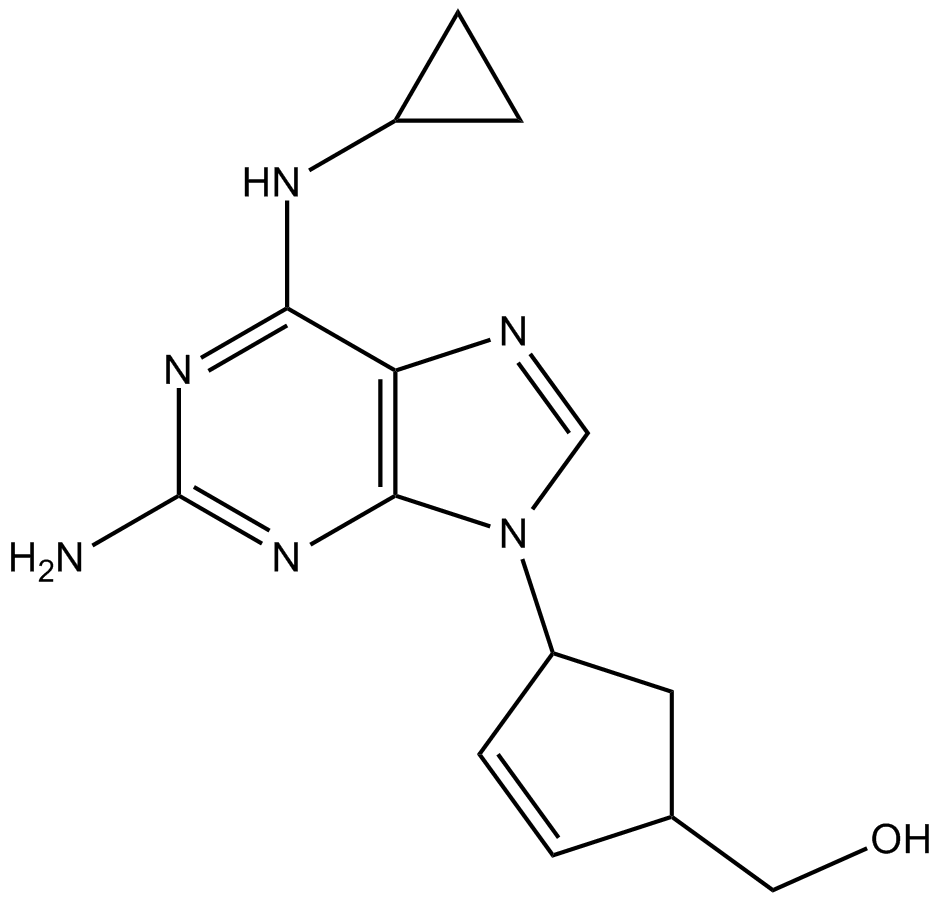
Cas No.: 136470-78-5
Sample solution is provided at 25 µL, 10mM.
IC50 Value: 0.26 microM for HIV-1[1] Abacavir,(-)-(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol, is a novel purine carbocyclic nucleoside analogue that has been approved by the FDA for the treatment of HIV (as Ziagen trade mark [abacavir sulfate]) [2]. in vitro: In erythrocytes, abacavir influx was rapid, nonsaturable (rate constant=200 pmol/s/mM/microl cell water), and unaffected by inhibitors of nucleoside or nucleobase transport[2]. in vivo: pharmacokinetic, distribution, and toxicological profiles of 1592U89 were distinct from and improved over those of CBV, probably because CBV itself was not appreciably formed from 1592U89 in cells or animals (<2%). The 5'-triphosphate of CBV was a potent, selective inhibitor of HIV-1 RT, with Ki values for DNA polymerases (alpha, beta, gamma, and epsilon which were 90-, 2,900-, 1,200-, and 1,900-fold greater, respectively, than for RT (Ki, 21 nM). 1592U89 was relatively nontoxic to human bone marrow progenitors erythroid burst-forming unit and granulocyte-macrophage CFU (IC50s, 110 microM) and human leukemic and liver tumor cell lines[1]. Clinical trial: Estimate The Effect Of Lersivirine On The Pharmacokinetics Of Abacavir + Lamivudine In Healthy Subjects. Phage1
Review for Abacavir
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
Review for Abacavir
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















