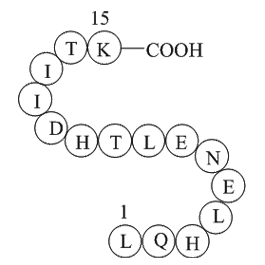
alpha-1 antitrypsin fragment (Synonyms: H2N-Leu-Gln-His-Leu-Glu-Asn-Glu-Leu-Thr-His-Asp-Ile-Ile-Thr-Lys-OH ) |
| Catalog No.GP10015 |
Protease inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
Alpha-1 antitrypsin (A1AT) (H2N-Leu-Gln-His-Leu-Glu-Asn-Glu-Leu-Thr-His-Asp-Ile-Ile-Thr-Lys-OH), which is known as serum trypsin inhibitor, is a protease inhibitor belonging to the serpin superfamily.
A1AT is a single-chain glycoprotein consisting of 394 amino acids in the mature form and exhibits a number of glycoforms. The three N-linked glycosylations sites are mainly equipped with so-called diantennary N-glycans. However, one particular site shows a considerable amount of heterogeneity since tri- and even tetraantennary N-glycans can be attached to the Asparagine 107 (ExPASy amino acid nomenclature).
A1AT is a major inhibitor of human serine proteases in serum, is produced mainly by the liver, but also by extrahepatic cells, including neutrophils and certain cancer cells. [1] AAT is an acute phase protein and its concentration rises up to 3-4-fold above normal during acute inflammation. [2] Several types of cancer, including non-small cell lung adenocarcinoma, have been associated with increased serum levels of AAT. [3] Clinical studies have shown that high circulating levels of AAT directly correlate with tumor progression. Recently it was also found that plasma levels of AAT are significantly elevated in lung cancer patients and, particularly in cases with metastases. [4] Moreover, not only the native, inhibitory form of AAT, but also conformationally modified, non-inhibitory forms are suggested to play a role in modulating tumor growth and invasiveness. For example, Kataoka and co-workers have shown that the C-terminal fragment of AAT can enhance the growth and invasiveness of human pancreas adenocarcinoma cells, in vivo [5].
References:
1. Paakko P, Kirby M, du Bois RM, Gillissen A, Ferrans VJ, Crystal RG: Activated neutrophils secrete stored alpha 1-antitrypsin. Am J Respir Crit Care Med 1996, 154:1829-1833.
2. Molmenti EP, Ziambaras T, Perlmutter DH: Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem 1993, 268:14116-14124.
3. Kataoka H, Itoh H, Koono M: Emerging multifunctional aspects of cellular serine proteinase inhibitors in tumor progression and tissue regeneration. Pathol Int 2002, 52:89-102.
4. Zelvyte I, Wallmark A, Piitulainen E, Westin U, Janciauskiene S: Increased Plasma Levels of Serine Proteinase Inhibitors in Lung Cancer Patients. Anticancer Res 2004, in press.
5. Kataoka H, Uchino H, Iwamura T, Seiki M, Nabeshima K, Koono M: Enhanced tumor growth and invasiveness in vivo by a carboxyl-terminal fragment of alpha1-proteinase inhibitor generated by matrix metalloproteinases: a possible modulatory role in natural killer cytotoxicity. Am J Pathol 1999, 154:457-468.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















