DOXORUBICIN
Dox or DOX is the short form of Doxorubicin, which is also called as Adriamycin,
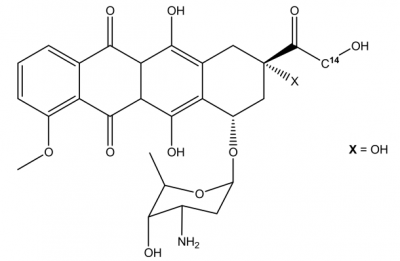
The molecular structure of Doxorubicin is diagrammatically depicted in Figure 1. In its molecular structure, there is a tetracyclic quinoid aglycone adriamycinone namely 14-hydroxydaunomycinone that is chemically linked to the amino- sugar namely daunosamine. Chemically, it is a derivative of Anthracycline that has antibiotic as well as anticancer properties. The amino group along with the quinone moiety present in the molecular structure of Doxorubicin are the main source of determining the anti-cancer properties of Doxorubicin.
PLX 3397
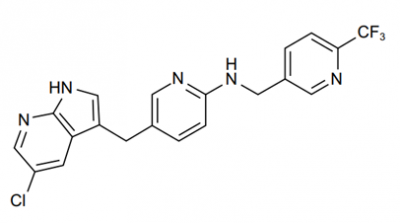
PLX-3397 is the shortened form of Plexxikon 3397, which is also called as Pexidartinib hydrochloride, TURALIO or simply Pexidartinib. Chemically, PLX-3397 is a small molecule. The molecular structure of the PLX-3397 is diagrammatically depicted in Figure 1.
Leu 15 Gastrin 1 Human
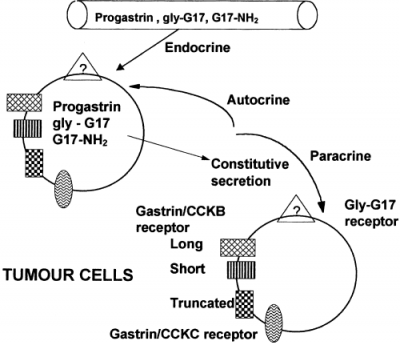
There are various names for the [Leu 15] -Gastrin 1 (Human); these names include human heptadecapeptide, [Leucine15]-Little gastrin I, [Leu15]-HG-17 and also LHG-17.
Nivolumab
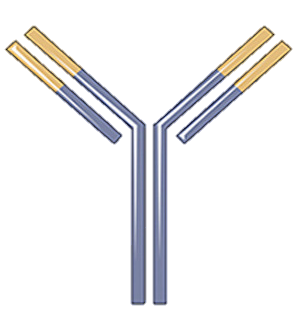
Nivolumab that is also called as MDX 1106, BMS 936558, ONO 4538 and OPDIVO; it is a compound that is produced in the laboratory by exploiting the genetic engineering technology. Chemically, it is globin protein that either enhances the performance or supports immune system; thus, it is regarded as an antibody or otherwise immunoglobulin. Nivolumab follows the generalized structure of an immunoglobulin; that is shown in Figure 1. More specifically, it belongs to the Immunoglobulin G4 (IgG4) subclass of the class Immunoglobulin G (IgG). It can specifically bind to its ligand that is programmed death-1 (PD-1) receptor protein with a high affinity and forms a molecular complex; because its nature of being monoclonal. The crystal structure of this molecular complex is diagrammatically presented in Figure 2. while the surface representation of this molecular complex is diagrammatically presented in Figure 3.
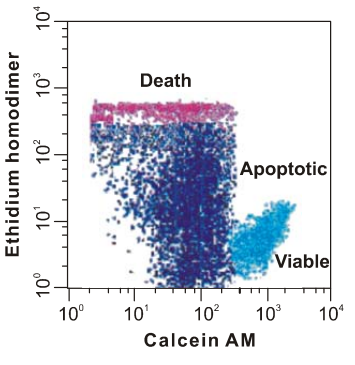
Calcein AM
Calcein AM is the short form of the calcein-acetoxymethyl ester, which is basically a non-fluorescent and cell permeant, i.e., that essentially means that it does not exhibit fluorescence and also the cell membrane allows its free movement across itself owing to its lipophilic nature. Chemically, it is a acetoxymethyl ester of calcein. It passively enters the cell, without any energy expenditure through a gap junction, where it gets biochemically converted, i.e., via the biochemical hydrolysis that is catalyzed by the activity of the esterase enzymes that reside in the cytoplasm, into Calcein, which is acidic in nature as it holds a negative charge being an anion. Moreover, the latter is cell-impermeant, i.e., which essentially means that it does not have the ability to come out of the cell and thus get trapped inside the cell. Furthermore, the important point is that the latter one is fluorophore. The key difference between how Calcein AM and Calcein interact with the cell is shown in Figure 1.
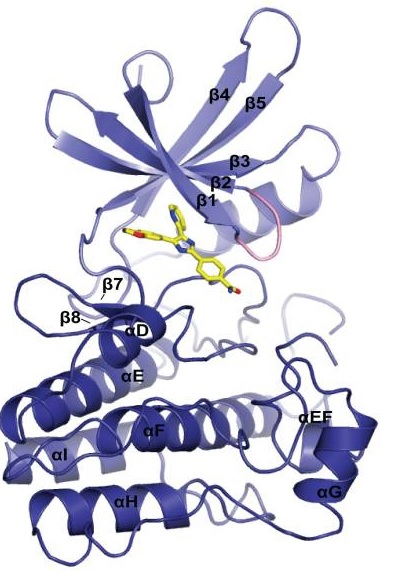
SB 431542
SB 431542 is a small molecule that forms a complex with TβRI Kinase domain. The structure of theTβRI Kinase domain-SB 431542 complex is diagrammatically shown in Figure 1 while the close interactions within this complex are diagrammatically illustrated in Figure 2.
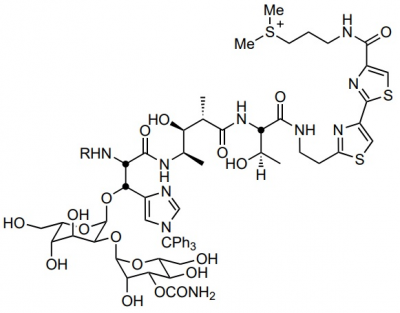
Bleomycin Sulphates
Bleomycin Sulphate, which is also known as bleomycin A1, bleomycin B2 or simply bleomycin. It belongs to a bleomycin, a class of glycopeptides having antitumor and antibiotic properties. Its molecular structure is diagrammatically depicted in Figure 1, while the four subunits or domains are highlighted in Figure 2. The complete process of its synthesis is well known and fully documented in the scientific literature in detail. Each of these domains has a unique function which has a biological role.
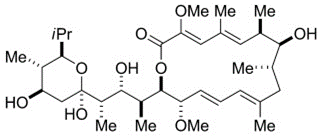
Bafilomycin A1
Bafilomycin A1 (Baf A1) is a macrolide with the antibiotic properties. It specifically and effectively inhibits the V-type ATPase. The absolute stereochemistry is also determined via a x-rays crystallographic study and its molecular structure is diagrammatically depicted in Figure 1. The complete process for its synthesis is well documented in detail in scientific literature.
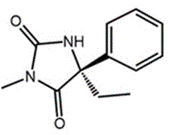
S-Mephenytoin
Chemically, Mephenytoin is 3-methyl-5, 5-phenyl-ethyl-hydantoin that is also known as Mesantoin. Mephenytoin is a molecule, that can be clinically used as a pharmaceutically active drug therapeutic drug. For any pharmaceutically active drug, its structural features are the single primary factor that not only determines the site of biochemical transformation during metabolism but also the rate of that biochemical transformation. The molecular structure of Mephenytoin is shown in Figure 1, while the existence of a chiral carbon at carbon number 5 and its chemical implications in terms of existence of Mephenytoin as a racemic mixture, are diagrammatically demonstrated in Figure 2.
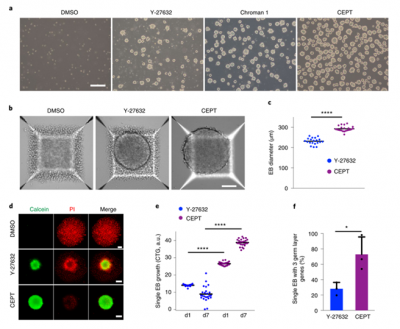
CEPT
Human pluripotent stem cells (HPSCs) have the ability to self-renew, but are highly sensitive to environmental disturbances in vitro, posing a challenge for their therapeutic applications. Further breakthroughs are urgently needed to ensure the safe and stable long-term growth and functional differentiation of these cells. Here, a high-throughput screening strategy is used to identify a small molecule mixture that can enhance the survival ability of hPSCs and their differentiated offspring. The combination of Chroman 1, Emricasan, Polyamines, and trans-ISRIB (CEPT) improves the cell survival rate of genetically stable hPSCs by simultaneously blocking several stress mechanisms that originally damage cell structure and function. CEPT provides powerful improvements for several key applications in stem cell research, including conventional cell passage, cryopreservation of pluripotent stem cells and differentiated cells, embryoid body (EB) and organoid formation, single cell cloning, and genome editing. Therefore, CEPT represents a unique multi pharmacological strategy for comprehensive cell protection, providing a theoretical basis for the efficient and safe utilization of hPSCs.