BODIPY 493/503
BODIPY 493/503 (4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-SIndacene) usually employed for the quantification of neutral lipid content by utilizing flow cytometry and for the observation of lipid droplets by microscopy, is a fluorescent and a neutral lipid. BODIPY 493/503 is a small molecular dye with strong ultraviolet absorption capability showcasing a sharp fluorescence peak with certainly no sensitization to the environment’s pH, polarity, and other physiological conditions. BODIPY, the common name that symbolizes boron-dipyrromethene, consists of a boron-difluoride group joined with a dipyrromethene group and is a red crystalline solid that is stable at subtle temperatures.
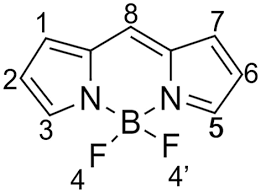
Fig Structure of BODIPY 493/503
BODIPY 493/503, succinimidyl ester form is exploited for conjugating hydrophobic proteins or lipids. The localization of the polar lipids in the cell is possible when BODIPY lipid droplets, specifically stain lipid droplets when dye passes through the cell membrane into the cell. Now, these stained lipid droplets can serve as markers for liver cells. BODIPY 493/503 is applied to discern oil and non-polar droplets in the liver or fixed cells, also utilized as lipophilic probes for polar and neutral lipids, with an excitation peak at 493 nm and emission peak at 503 nm.
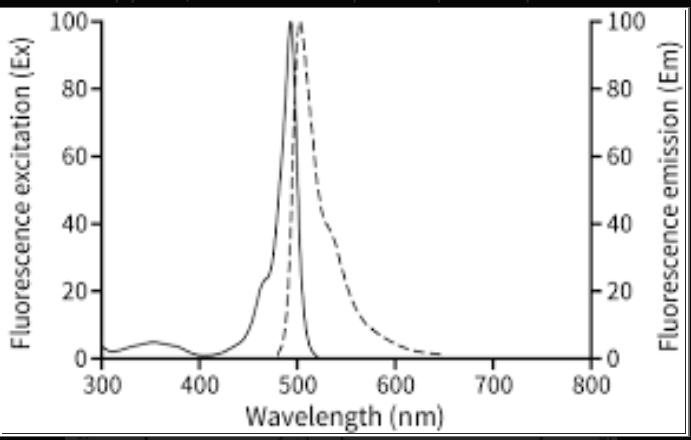
Fig demonstrating the excitation and emission peak of BODIPY 493/503
A study focusing on exploring the impact of hydrophobic-hydrophilic drug interactions on dual drug release from Co2-infused scaffolds by employing BODIPY dyes as they are quite remarkable and distinguished because of the high solubility in organic solvents, brightness, quantum yields, and uniquely small stock shifts. The fluctuation of solvent polarity does not affect the emission and excitation of BODIPY 493/503.
BODIPY dyes are evident for their peculiarly small stoke shifts, precise absorption bands, fine fluorescence peaks, and high quantum yields with outstanding chemical and photostability characteristics. BODIPY fluorophores are used as compelling tools in a variety of applications like biochemical labeling, light-emitting devices, supramolecular fluorescent gels, and sensitization in solar cells with the combination of these extraordinary attributes.
Triacylglycerol (triglyceride, or TAG) stored form generally contains the highest substantial amounts in white adipose tissue and is stored within organelles named neutral lipid droplets (LDs). Previous studies have shown that significant lipid droplet accumulation can occur in various tissues, such as skeletal muscles, under subtle conditions whose appearance in nonadipose tissue is referred to as ectopic fat. Several clinical conditions interrelated with the increment in the amount of ectopic fat include peripheral insulin resistance and muscular dystrophy. Therefore, there is a need to measure or visualize the accumulation of stored TAG in the form of lipid droplets in skeletal muscle. To visualize LDs within skeletal muscle fibers, we analyze the use of BODIPY 493/503 to resolve flexibility issues by implying a variety of different conditions.
Following are the methods and guidelines one can opt for on how to employ BODIPY 493/503 for the visualization of intramuscular lipid droplets in skeletal muscles. Firstly, we have to remove multiple skeletal muscles from the designated animal which we are going to use for visualizing lipid droplets and is approved by the Animal Care and Use Committee at the University of Maryland for procedure. After the isolation of skeletal muscles from animals, it is frozen in liquid nitrogen-cooled isopentane or stored at −80◦C, followed by cutting of transverse sections on a cryostat (10 µm thick) and ultimately collected onto glass slides. For single fiber isolation, muscles were incubated in dissociation media and then incubated (37◦, 5% CO2) for 2 hours. After the dissociation to yield single myofibres, muscles were placed in a new 35 mm plate with fresh media and triturated with a small bore (∼1 mm) fire-polished glass transfer pipette. The fibers are then plated onto extracellular matrix-coated dishes and are allowed to incubate overnight before BODIPY staining or immunolabeling. To influence LD formation in fibers, the dishes have to be incubated overnight in a high-fat media containing equal molar concentrations of palmitate (PA) and oleate (OA) without any toxicity threats.
BODIPY 493/503 is diluted in PBS or DMSO with a concentration of 1 mg/mL and applied to cross sections or fibers for 30 mins correspondingly. The samples are washed about three times in phosphate-buffered saline (PBS) for 10 min after fixation. Some cross-sections are stained with 5ug/mL rhodamine-labeled succinylated wheat germ agglutinin, while others are labeled with antibodies to identify slow (Type I) muscle fibers.
Sections of muscle fibers were then subjected to rinsing for 10 minutes in 100 mmol glycine-buffered saline (glycine PBS). Then they tended to be barred in 1% bovine serum albumin PBS, followed by their incubation with primary antibodies for straight 2 hours in diluted 22 mg/mL PBS. Before the incubation of tissue sections with species-specific secondary antibodies, it was priorly exposed to 3 times washing with 1% BSA/PBS for 10 minutes. After this step, samples were cloaked with glass coverslips and secured in VECTASHIELD (Vector Laboratories). By employing Zeiss 510 confocal laser scanning microscope mins, the digital images of samples were obtained when they were examined under epifluorescent optics.
For the examination of the left samples, serial sections (10 µM) from frozen muscles were taken into account with the fixation of samples with formalin for 5 minutes and spreading of them onto a glass slide which was then followed by the rinsing of samples with tap water. Following the procedure, slides were stained with filtered 5% oil red solution for 30 minutes after being subjected to the washing of slides with 60% isopropanol. The samples were then secured in VECTASHIELD (Vector Laboratories).
To conclude, BODIPY has offered us a promising alternative for the visualization of lipid droplets in live and fixed cells, bypassing traditional staining methods. Moreover, its amazing characteristics of versatility, brightness, and minimal toxicity have pushed this method of staining into a valuable tool for researchers exploring the scientific realm, particularly for comprehending numerous aspects of cellular metabolism and function. More research on this powerful tool can solidify its place in the field of cell biology to obtain improve and optimize results.













Comments