Lipopolysaccarides
LPS from members of the Enterobacteriaceae family has undergone structural analysis (Fig. 1). There have been three distinct regions described: The O-specific antigen, also known as the O-antigen or somatic antigen, is a long-chain polysaccharide made up of repeating units that contain one to seven sugars, or monosaccharides (ii) the oligosaccharide core, which is made up of roughly ten monosaccharides (including 3-keto-2-deoxyoctonate [KDO], heptose, glucose, galactose, and N'-acetylglucosamine), which are added successively by unique glycosaccharide transferases; and (iii) lipid A, a distinct lipid backbone made up of diglucosamine in p1-6 linkage with fatty acids that are ester-, amide-, and diester-linked (the type and position of fatty acids vary among species) and with phosphate, 4-amino-arabinose substituents, orboth on the reducing and nonreducing sugars. Lipid A is released from the core and the 0-antigen polysaccharide by mildly acidic hydrolysis procedures, which also produce damaged polysaccharide and free lipid A. Free lipid and insoluble form (electrodialyzed saltform) The majority of the biochemical and endotoxic traits of intact LPS are present in A.
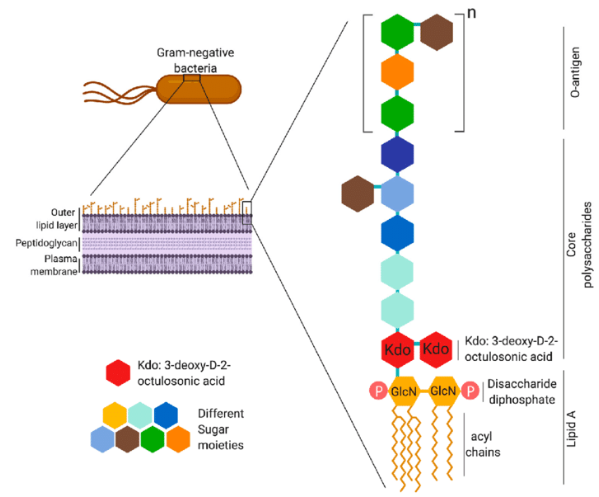
Figure 1: Overall structure of Lipopolysaccrides.
When a bacterial cell is living in the gastrointestinal system, its main job is to maintain structural integrity and act as a barrier against the introduction of harmful substances like toxins and bile salts. The acyl chain interacts extensively when there are several saturated fatty acid moieties present, which reduces the fluidity of the membrane bilayer. Divalent cations, including magnesium, intercalate between LPS molecules to sustain the membrane's high negativity caused by the phosphate group. LPS packing is improved by the final structure created by polyionic interactions, which ultimately turns LPS into a structural barrier to the bacterial cell. Furthermore, the main factor that gives the bacterial cell its pathogenicity is LPS. Endotoxins (LPS) are relatively stable in comparison to typical bacterial exotoxins and are the main biological response modifiers that cause particular disease pathologies and symptoms. The host immune cells are strongly and pleiotropically stimulated by LPS. The lipid is recognized by the immune system. a part of LPS that is released in soluble form from proliferating cells, autolyzed bacterial cells upon autolysis, or bacteria killed by phagocytosis, complement activation, or antibiotic action. The lipid A component is recognized and binds to the host cell's TLR4 to initiate the main reaction. Due to the wide variation in Lipid A's composition, different bacterial strains elicit varying degrees of immunological response. While Yersinia pestis and other bacteria create LPS with minimal immunogenicity in vivo by modulating the degree of lipid A's acylation during infection, lipid A produced by E. coli and Salmonella is highly immunogenic. It's interesting to note that bacteria use the synthesis of a low-immunogenic LPS as a tactic to boost intracellular survival and elude the host immune system .
Within the system of the host, the lipid The receptor on the surface of macrophages and endothelial cells detects a moiety of LPS at picomolar levels. Firstly, LPS attaches itself to serum LBP and then transmits it to the CD14 receptor on immune cell membranes. It is transferred by the CD14 to the non-anchored protein MD2, which then engages in interaction with TLR4. Consequently, the CD14/TLR4/MD2 receptor complex, which is found in a variety of host cell types including monocytes, dendritic cells, macrophages, and B cells, binds to LPS. The sort of cell to which LPS is coupled determines the next course of action . Three conceivable reactions, listed below, are elicited in monocytes and macrophages;
1. Cytokine production, including TNF, IL-1, IL-6, and IL-8, which promotes the release of prostaglandins and leukotrienes, ultimately resulting in the inflammation and septic shock that are the main characteristics of endotoxemia.
2. Histamine release brought on by complement activation causes vasodilation along with neutrophil chemotaxis and accumulation.
3. Coagulation cascade activation: Blood-clotting factor XII causes thrombosis, acute disseminated intravascular coagulation, and coagulation by activating humoral systems. Hemorrhage and fibrinolysis are caused by plasmin activation, whereas inflammation is caused by activation of the alternative pathway. Bradykinins and other vasoactive peptides are released when kinin is activated, which results in hypotension. Ultimately, this results in the development of septic shock, intravascular coagulation, bleeding, and inflammation .
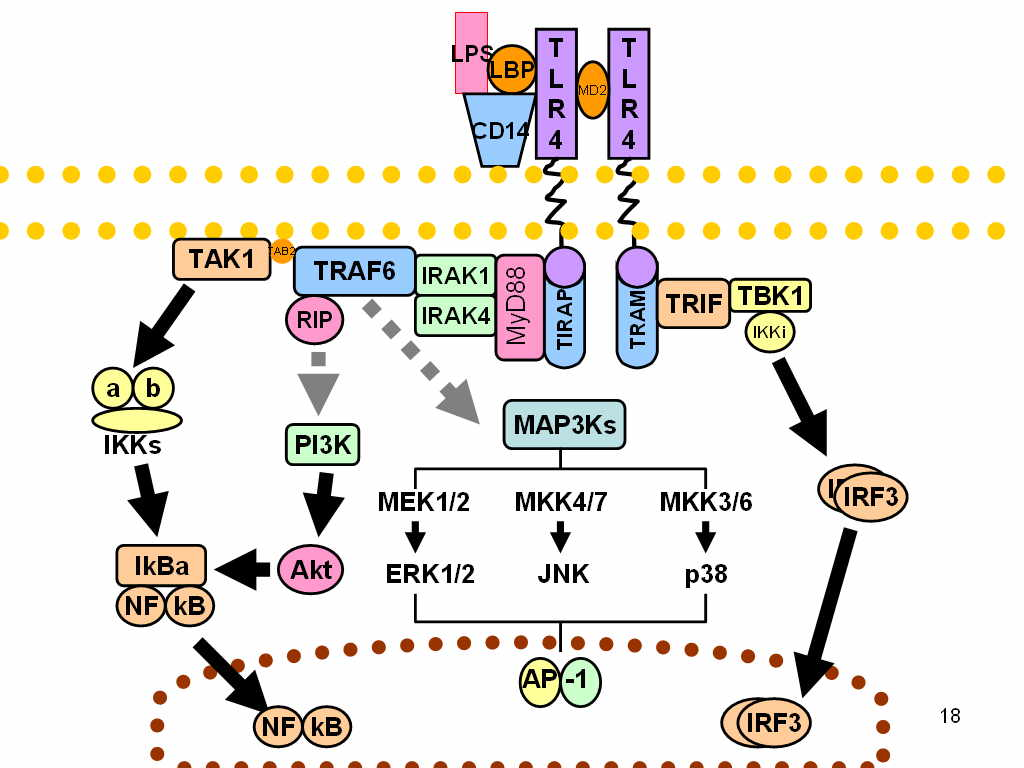
Figure 2: Mechanism of action of lipopolysaccrides.
Strong endotoxins like lipopolysaccharides (LPS) attach to cell surface receptors including TLR4/CD14/MD2, causing the release of pro-inflammatory cytokines, nitric oxide, and eicosanoids. Through the Lipid A moiety, the presence of LPS in the blood or interstitial fluid can produce endotoxemia, which under extreme immune reaction might result in septic shock. In addition to tachycardia and tachypnea, septic shock also involves temperature changes and the activation of the coagulation cascade, which causes venous and arterial dilatation . The ensuing hypovolemia causes insufficient tissue perfusion, which causes cellular malfunction. Lately, autoimmune disorders and allergies have been linked to modest doses of LPS exposure, while metabolic syndrome is caused by high blood concentrations of LPS. This raises the possibility of developing dangerous conditions like type 2 diabetes, heart problems, and liver problems. Moreover, endotoxins are intrinsically linked to the clinical presentations of infections with Gram-negative bacterial pathogens, such Neisseria meningitides, which result in meningococcal illness, including meningitis, meningococcemia, and Waterhouse-Friderichsen syndrome. Through LPS structure-function changes, certain opportunistic infections, such as Salmonella enterica, Helicobacter pylori, Pseudomonas aeruginosa, and Burkholderia cepacia complex bacteria, can adapt and cause a chronic infection in the gastrointestinal and respiratory tracts. Recent studies have shown that lipoprotein metabolism, membrane apo E/amyloid-beta interactions, and cholesterol-interacting proteins are all impacted by the membrane lipid disruptions that LPS causes. These changes put one at risk for dyslipidemia, hypercholesterolemia, and non-alcoholic fatty liver disease . There are instances where LPS can obstruct the body's ability to eliminate toxins, which has been connected to neurological deterioration .
LPS is a useful marker for early infection diagnosis. As little as 1 to 2 mg of LPS in the serum can cause toxicity in the host, primarily through the lipid A component (the endotoxin). Endotoxins can cause inflammation, fever, leukopenia, and blood vessel damage, which can ultimately result in hypotension. Suction and septicemia can result from high endotoxins. Low dosages of lipopolysaccharide (LPS) can aid in immunological regulation by promoting polyclonal differentiation and B-cell proliferation that results in the secretion of antibodies, particularly IgG and IgM. LPS from the stomach helps the immune system grow in newborns. While Bacteroides LPS induces food allergies and anti-insulin antibodies, which are early indicators of immunological dysfunction, Bifidobacterium LPS is beneficial during the development of a balanced immune system. Low concentrations of endotoxin, which are included in vaccines and other immunotherapeutics, can induce TLR activation, improving vaccination and medication efficacy and improving therapeutic results. Adjuvants with low levels of lipopolysaccharides (LPS) can function more actively. Thus, innate immune response modulation impurities (IIRMIs) is the term for it .
LPS preparations help clarify LPS production, metabolism, immunology, physiology, and toxicity in clinical studies. In both in vitro and in vivo research investigations, LPS is also utilized to stimulate the production and secretion of growth-promoting proteins like interleukins. Since LPS directly contributes to septicemia, it has been investigated to find potential antibody targets and inhibitors. Further research may lead to a better understanding of LPS, which could lead to innovative methods for developing antibacterial drugs and improve medical knowledge.













Comments