Cytochalasin D (Synonyms: NSC 209835) |
| Catalog No.GC13440 |
An inhibitor of actin polymerization, selective
Products are for research use only. Not for human use. We do not sell to patients.
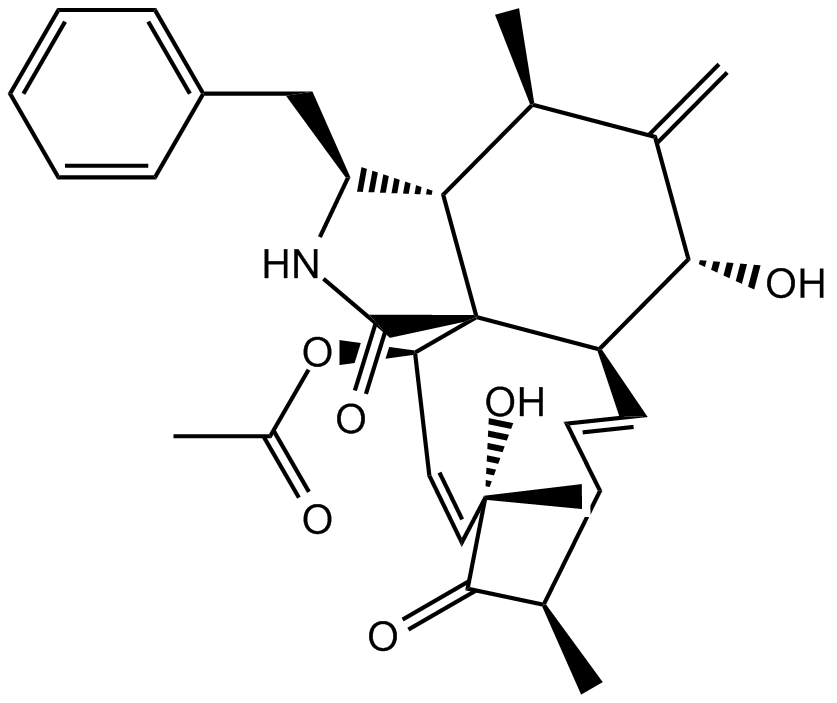
Cas No.: 22144-77-0
Sample solution is provided at 25 µL, 10mM.
The cytochalasins are cell-permeable fungal metabolites that inhibit actin polymerization.[1],[2],[3],[4] This interferes with such diverse processes as cell movement, growth, phagocytosis, degranulation, and secretion.[5],[6],[7],[8] Cytochalasin D is a cell-permeable inhibitor that binds actin filaments, but not actin monomers, to inhibit polymerization at concentrations as low as 0.2 µM.2 In this way, it prevents the migration of tumor cells.[9]
Reference:
[1]. Brenner, S.L., and Korn, E.D. The effects of cytochalasins on actin polymerization and actin ATPase provide insights into the mechanism of polymerization. The Journal of Biological Chemisty 255(3), 841-844 (1980).
[2]. Lin, D.C., Tobin, K.D., Grumet, M., et al. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. Journal of Cell Biology 84, 455-460 (1980).
[3]. Ostlund, R.E., Jr., Leung, J.T., and Hajek, S.V. Regulation of microtubule assembly in cultured fibroblasts. Journal of Cell Biology 85, 386-391 (1980).
[4]. Pinder, J.C., and Gratzer, W.B. Structural and dynamic states of actin in the erythrocyte. Journal of Cell Biology 96(3), 768-775 (1983).
[5]. Flaumenhaft, R., Dilks, J.R., Rozenvayn, N., et al. The actin cytoskeleton differentially regulates platelet α-granule and dense-granule secretion. Blood 105(10), 3879-3887 (2005).
[6]. Taheri-Talesh, N., Horio, T., Araujo-Bazán, L., et al. The tip growth apparatus of Aspergillus nidulans. Molecular Biology of the Cell 19, 1439-1449 (2008).
[7]. dos Santos, T., Varela, J., Lynch, I., et al. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS One 6(9), 1-10 (2011).
[8]. Nightingale, T.D., White, I.J., Doyle, E.L., et al. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. Journal of Cell Biology 194(4), 613-629 (2011).
[9]. Hayot, C., Debeir, O., Van Ham, P., et al. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicology and Applied Pharmacology 211, 30-40 (2006).
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




