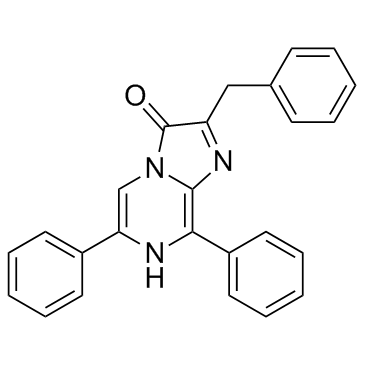
Diphenylterazine (DTZ) (Synonyms: DTZ) |
| Catalog No.GC33428 |
Diphenylterazine (DTZ) (DTZ) is a bioluminescence agent.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 344940-63-2
Sample solution is provided at 25 µL, 10mM.
Diphenylterazine, an analog of CTZ, is a good luciferase substrate. Diphenylterazine is a bioluminescence agent. Diphenylterazine alone yields very little background, leading to excellent signal-to-background ratios. Furthermore, Diphenylterazine elicits minimal cell toxicity at millimolar concentrations. Diphenylterazine could be synthesized from inexpensive commercial reagents in two steps with excellent yields[1].
In cell experiments, we can see that Diphenylterazine has a lower signal-to-noise ratio than other substrates, and Diphenylterazine causes minimal cytotoxicity at milimolar concentrations.
In contrast, other tested substrate induced cell death within the tested substrate concentration range.
In the mouse experiment, the bioluminescence of the surface site, at 0.1 mM substrate concentration, Diphenylterazine brightness was higher than that of the control group.
The same was true for imaging of deep tissue targets, and Diphenylterazine injection into untransfected BALB/c mice did not produce any background emission, and fluorescence was still detectable when injected intravenously into mice[2].
References:
[1].Tian X, Zhang Y, Li X, Xiong Y, Wu T, Ai HW. A luciferase prosubstrate and a red bioluminescent calcium indicator for imaging neuronal activity in mice. Nat Commun. 2022 Jul 8;13(1):3967. doi: 10.1038/s41467-022-31673-x. PMID: 35803917; PMCID: PMC9270435.
[2]. Yeh HW, et al. Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging. Nat Methods. 2017 Oct;14(10):971-974.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















