GW4869 |
| Catalog No.GC19186 |
GW4869 is a noncompetitive neutral sphingomyelinase(N-SMase) inhibitor with an IC50 of 1 uM.
Products are for research use only. Not for human use. We do not sell to patients.
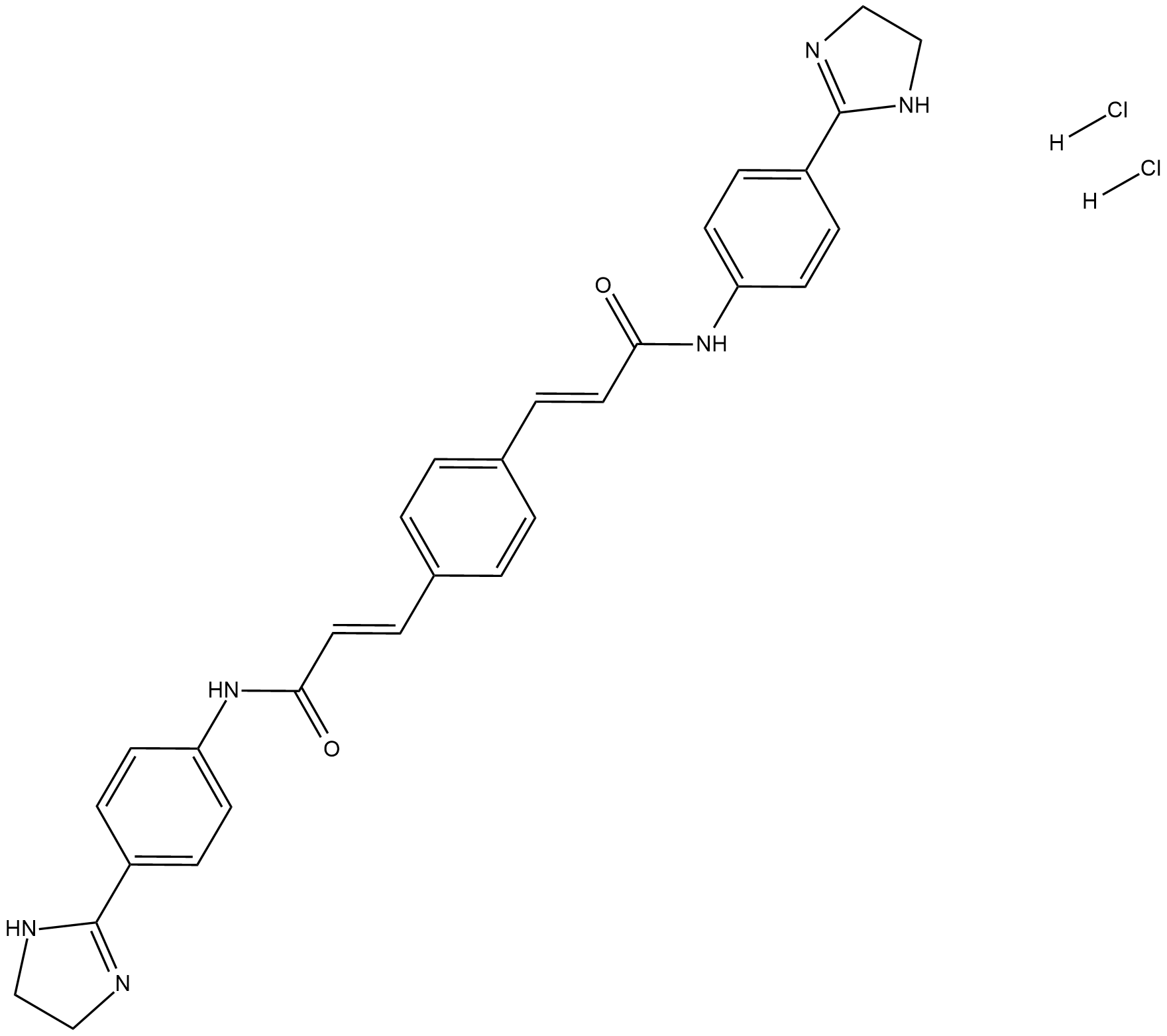
Cas No.: 6823-69-4
Sample solution is provided at 25 µL, 10mM.
GW4869 is a noncompetitive neutral sphingomyelinase inhibitor with an IC50 of 1 uM.
GW4869 (10 uM) partially inhibits TNF-induced sphingomyelin (SM) hydrolysis, and 20 uM of the compound is protected completely from the loss of SM. The addition of 10-20 uM GW4869 completely inhibits the initial accumulation of ceramide, whereas this effect is partially lost at later time points (24 h). The action of GW4869 occurs downstream of the drop in glutathione. GW4869 is able, in a dose-dependent manner, to significantly protect from cell death. These protective effects are accompanied by significant inhibition of cytochrome c release from mitochondria and caspase 9 activation, therefore localizing N-SMase activation upstream of mitochondrial dysfunction[1].
Pre-treatment with GW4869 significantly impairs release of both exosomes and pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in RAW264.7 macrophages. At 12 h after LPS treatment or CLP surgery, WT mice pretreated with GW4869 displays lower amounts of exosomes and pro-inflammatory cytokines in the serum than control PBS-injected mice. Accordingly, GW4869 treatment diminishes the sepsis-induced cardiac inflammation, attenuates myocardial depression and prolongs survival[2].
References:
[1]. Luberto C, et al. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutralsphingomyelinase. J Biol Chem. 2002 Oct 25;277(43):41128-39.
[2]. Essandoh K, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015 Nov;1852(11):2362-71.
[3]. Nakamura H, et al. Sphingomyelin Regulates the Activity of Secretory Phospholipase A2 in the Plasma Membrane. J Cell Biochem. 2015 Sep;116(9):1898-907.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




