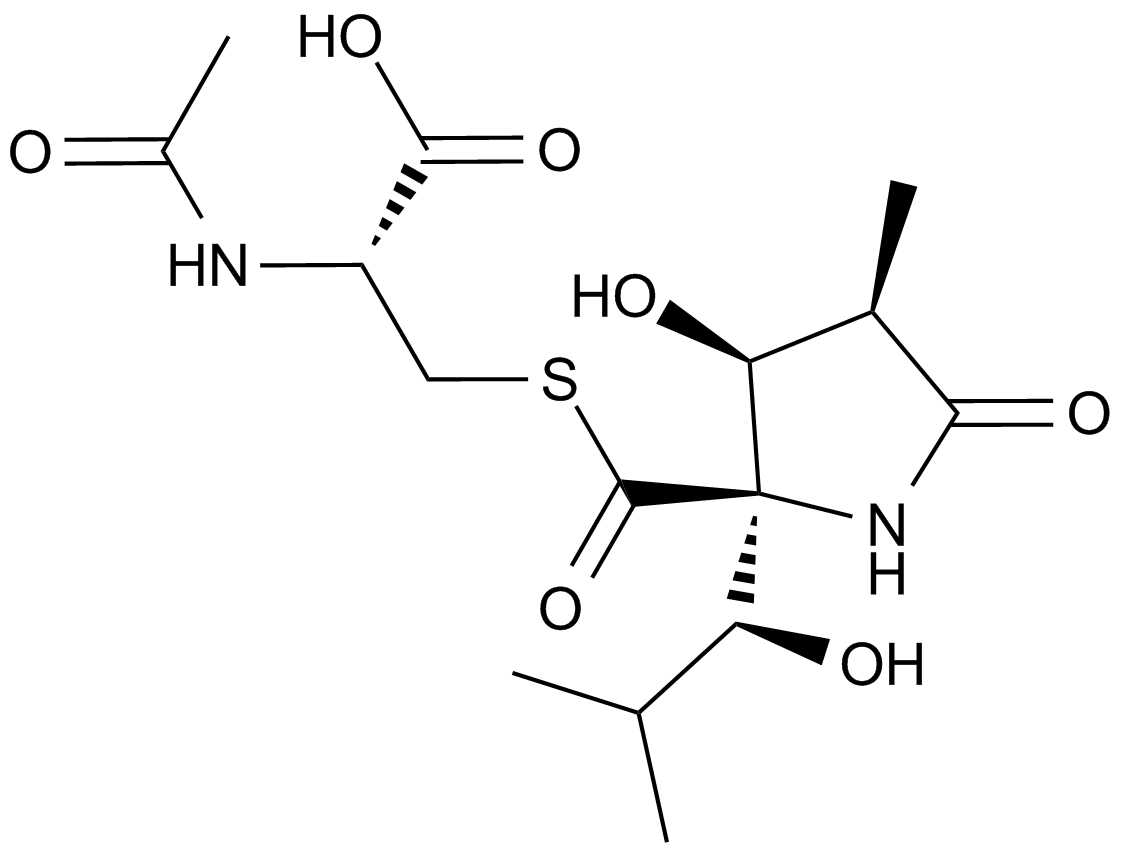
Lactacystin (Synthetic) |
| Catalog No.GC13123 |
A selective inhibitor of the 20S proteasome
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 133343-34-7
Sample solution is provided at 25 µL, 10mM.
Lactacystin is a microbial metabolite isolated from Streptomyces that is now widely used as a selective inhibitor of the 20S proteasome.[1],[2],[3] Lactacystin was first characterized by its ability to induce differentiation and inhibit cell cycle progression in several tumor cell lines. At concentrations from 2 to 10 μM, lactacystin induces the outgrowth of neurites in the neuroblastoma cell line Neuro2a.4 Lactacystin irreversibly alkylates subunit X of the 20S proteasome.[3] The concomitant inhibition of proteasome peptidase activity results in the accumulation of a variety of ubiquitinated proteins which would normally undergo rapid degradation. Thus, the effects of lactacystin are pleiotropic and depend substantially on the expression pattern of signalling proteins within the treated cell.
Reference:
[1]. Omura, S., Fujimoto, T.T., Otoguro, K., et al. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. Journal of Antibiotics 44, 113-116 (1991).
[2]. Corey, E.J., and Reichard, G.A. Total synthesis of lactacystin. Journal of the American Chemical Society 114, 10677-10678 (1992).
[3]. Fenteany, G., and Schreiber, S.L. Lactacystin, proteasome function, and cell fate. The Journal of Biological Chemisty 273(15), 8545-8548 (1998).
[4]. Fenteany, G., Standaert, R.F., Reichard, G.A., et al. A β-lactone related to lactacystin induces neurite outgrowth in a neuroblastoma cell line and inhibits cell cycle progression in an osteosarcoma cell line. Proceedings of the National Academy of Sciences of the United States of America 91, 3358-3362 (1994).
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




