R-7050 (Synonyms: TNF-α Antagonist III) |
| Catalog No.GC11659 |
R-7050 is a novel cell-permeable triazoloquinoxaline compound that selectively inhibited TNF-α induced cellular signaling.
Products are for research use only. Not for human use. We do not sell to patients.
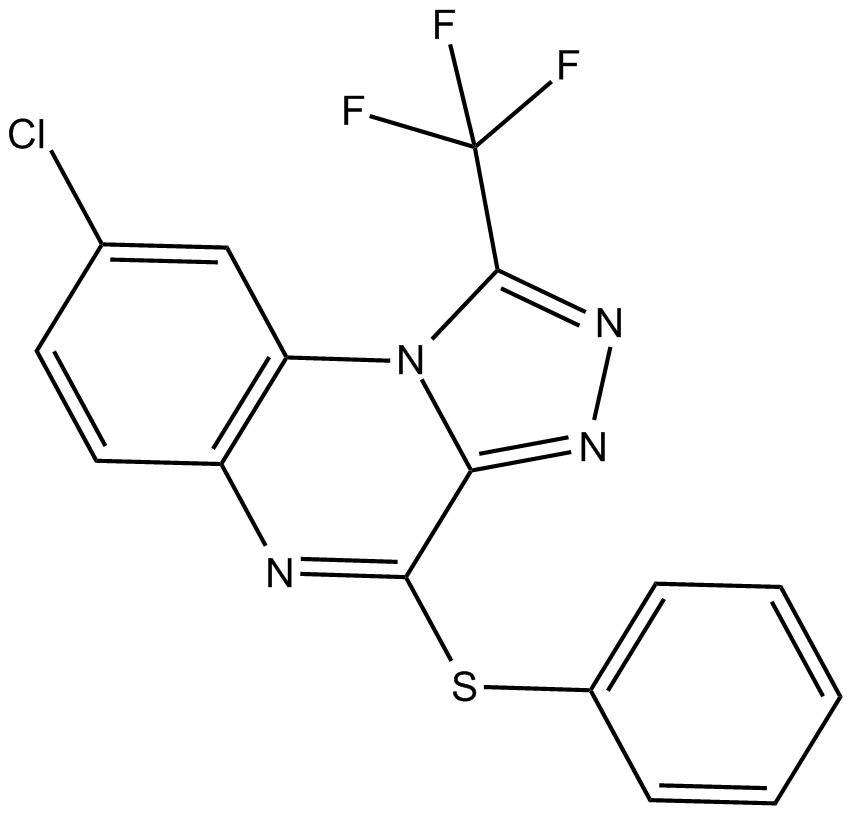
Cas No.: 303997-35-5
Sample solution is provided at 25 µL, 10mM.
R-7050 is a novel cell-permeable triazoloquinoxaline compound that selectively inhibited TNF-α induced cellular signaling[2].
In RBL-2H3 cells R-7050 significantly reduced TNF proliferation when autophagy was also inhibited by Baf-A1[7].CA to reduce cell viability in MDA-MB-231 breast cancer cells and tumorigenic HEK 293 cells but not in normal NIH3T3 fibroblast cells. Abundance of TNFA, TNF Receptor 1 (TNFR1) and cleaved caspase-8/-3 proapoptotic proteins to increase with CA treatment. Blocking of TNFA-TNFR1 signalling by small molecule inhibitor, R-7050, reduced the expression of cleaved caspase-8 and caspase-3 at the protein level[3]. TRPA1 channel was positively expressed in the cell bodies and processes of HODs. The expression TRPA1 channel was significantly up-regulated by high concentration of TNF-α, which could be suppressed by R-7050[5].
A single administration of R-7050 reduced neurovascular injury after ICH. In contrast to biologic approaches that directly bind and neutralize TNF-α activity, R-7050 selectively inhibits the association of TNFR with intracellular adaptor molecules, such as TRADD and RIP1, limiting receptor internalization and preventing subsequent cellular responses after TNF-α binding[1]. Capsaicin, a selective TRPV1 agonist, increased ipsilateral afferent renal nerve activity in WT but not TRPV1-/- mice. WD intake increased leptin, IL-6, and TNF-α in adipose tissue, and TNF-α antagonist III, R-7050, decreased leptin in TRPV1-/--WD. The urinary albumin level was higher in TRPV1-/--WD than WT-WD[4]. Treatment with the TNF receptor-1 inhibitor R-7050 during nephrotoxic serum nephritis reduced damage, fibrosis, and necroptosis in wild-type mice and mice with KLF4-deficient macrophages, and abrogated the differences between the two groups in these parameters[6].
References:
[1]. King MD, Alleyne CH Jr,et,al. TNF-alpha receptor antagonist, R-7050, improves neurological outcomes following intracerebral hemorrhage in mice. Neurosci Lett. 2013 May 10;542:92-6. doi: 10.1016/j.neulet.2013.02.051. Epub 2013 Mar 7. PMID: 23499961; PMCID: PMC3744337.
[2]. Gururaja TL, Yung S, et,al. A class of small molecules that inhibit TNFalpha-induced survival and death pathways via prevention of interactions between TNFalphaRI, TRADD, and RIP1. Chem Biol. 2007 Oct;14(10):1105-18. doi: 10.1016/j.chembiol.2007.08.012. PMID: 17961823.
[3]. Pal A, Tapadar P, Pal R. Exploring the Molecular Mechanism of Cinnamic Acid-Mediated Cytotoxicity in Triple Negative MDA-MB-231 Breast Cancer Cells. Anticancer Agents Med Chem. 2021;21(9):1141-1150. doi: 10.2174/1871520620666200807222248. PMID: 32767960.
[4]. Zhong B, Ma S, et,al. Ablation of TRPV1 Elevates Nocturnal Blood Pressure in Western Diet-fed Mice. Curr Hypertens Rev. 2019;15(2):144-153. doi: 10.2174/1573402114666181031141840. PMID: 30381083; PMCID: PMC6635649.
[5]. Liu J, Que K, et,al. Tumor Necrosis Factor-α Regulates the TRPA1 Expression in Human Odontoblast-Like Cells. J Pain Res. 2020 Jul 6;13:1655-1664. doi: 10.2147/JPR.S255288. PMID: 32753941; PMCID: PMC7352379.
[6]. Wen Y, Lu X, et,al. KLF4 in Macrophages Attenuates TNFα-Mediated Kidney Injury and Fibrosis. J Am Soc Nephrol. 2019 Oct;30(10):1925-1938. doi: 10.1681/ASN.2019020111. Epub 2019 Jul 23. PMID: 31337692; PMCID: PMC6779357.
[7]. Ayo TE, Adhikari P, et,al. TNF Production in Activated RBL-2H3 Cells Requires Munc13-4. Inflammation. 2020 Apr;43(2):744-751. doi: 10.1007/s10753-019-01161-4. PMID: 31897916; PMCID: PMC7176528.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




