SU5416 (Synonyms: NSC 696819, Semaxinib, Sugen 5416, VEGFR 2 Kinase Inhibitor) |
| Catalog No.GC15307 |
A tyrosine kinase inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 204005-46-9,194413-58-6
Sample solution is provided at 25 µL, 10mM.
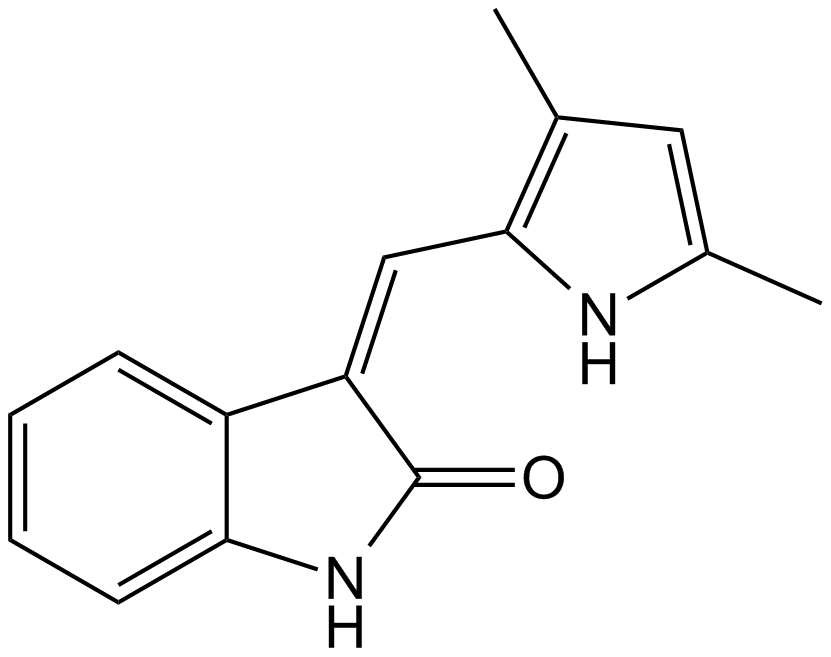
SU5416 is a potent small molecule vascular endothelial growth factor receptor (VEGFR) inhibitor. SU5416 is a 3-substituted indolin-2-one compound with relatively high specificity for VEGFR-2 and VEGFR-1, used extensively in animal models of PH, primarily due to effects on pulmonary vascular endothelial cell apoptosis and proliferation.[1] SU416 has been developed for the treatment of solid human tumors as well. [3]
In vitro study was performed to examine the inhibitory effect of SU5416 on KDR phosphorylation. Which indicated that pretreatment of BCECs with SU5416 resulted in a dose-dependent inhibition of KDR phosphorylation with an IC50 of 0.29 ± 0.071 μM (n=6) SU5416 almost completely inhibited KDR phosphorylation at the concentration of 3 μM. Few BCECs were stained with trypan blue after the treatment of SU5416, at least up to the concentration of 3 μM for 24 h. This suggested that the inhibitory effect of SU5416 on KDR phosphorylation was not due to the cell toxicity.[2]
In vivo study demonstrated that SU5416 could significantly reverse LPS-induced ALI in mice, and exert better protective effect in TLR4 knockout mice. SU5416 could also act as a protective agent against LPS-induced ALI in mice. Moreover, SU5416 dramatically restored the reduction of CD31 expression mediated by LPS, suggesting SU5416 could rescue LPS-induced dysfunction of pulmonary endothelial barrier. In addition, both p-VEGFR2 and VEGFR2 expressions were inhibited by SU5416 in WT and TLR4?/- mice. SU5416 could attenuate LPS-induced ALI through modulating the VEGF/VEGFR and NF-κB pathways, which suggested SU5416 might be used for the treatment of patients with inflammation-mediated ALI.[3]
References:
[1]. Peloquin GL, et al. SU5416 does not attenuate early RV angiogenesis in the murine chronic hypoxia PH model. Respir Res. 2019 Jun 17;20(1):123.
[2] Takeda A, et al. Suppression of experimental choroidal neovascularization utilizing KDR selective receptor tyrosine kinase inhibitor. Graefes Arch Clin Exp Ophthalmol. 2003 Sep;241(9):765-72.
[3]. Huang X, et al. SU5416 attenuated lipopolysaccharide-induced acute lung injury in mice by modulating properties of vascular endothelial cells. Drug Des Devel Ther. 2019 May 23;13:1763-1772.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




