Atosiban (Synonyms: RW22164; RWJ22164) |
| Catalog No.GC12194 |
mixed antagonist of oxytocin and vasopressin receptors
Products are for research use only. Not for human use. We do not sell to patients.
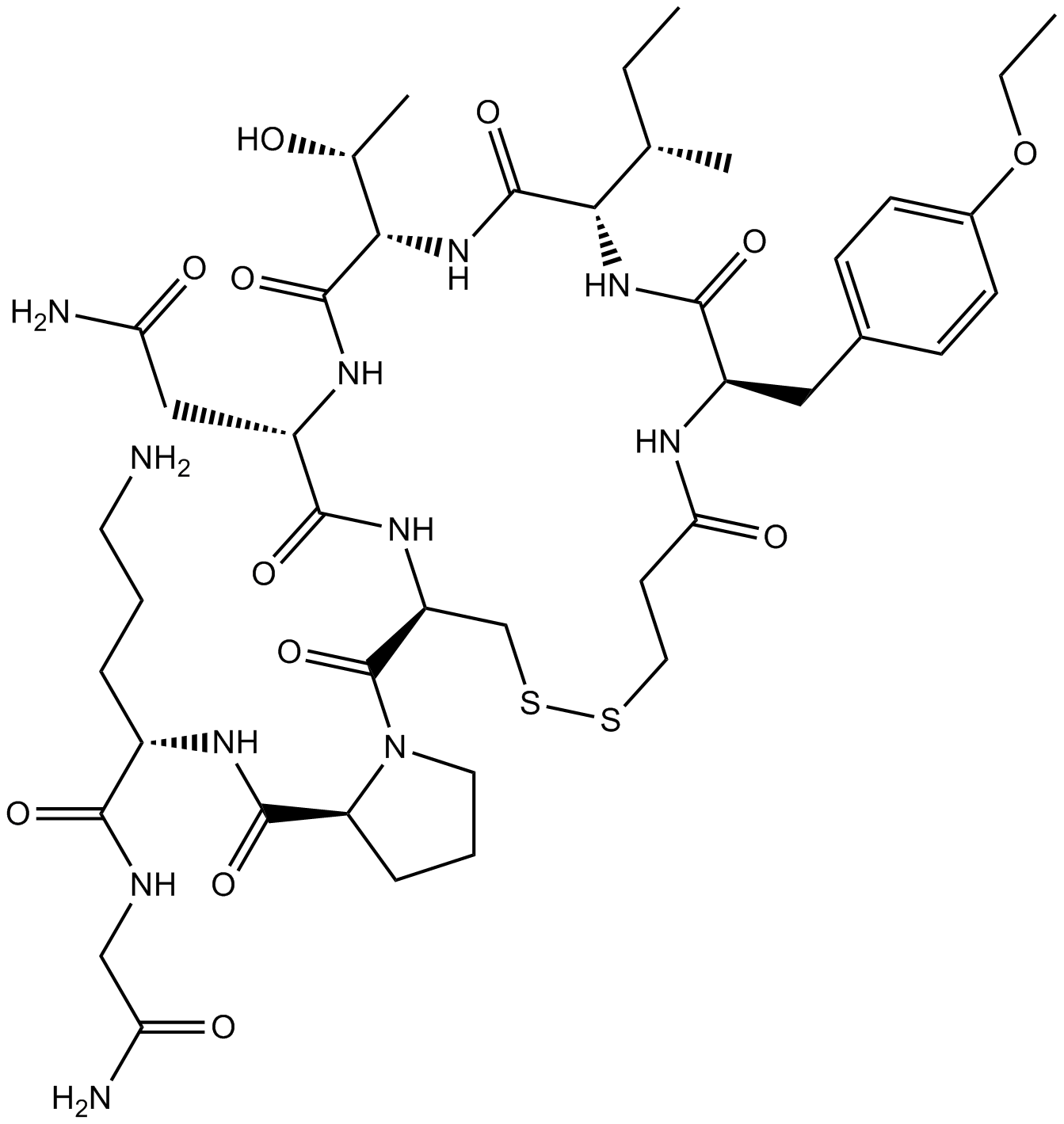
Cas No.: 90779-69-4
Sample solution is provided at 25 µL, 10mM.
Atosibana is a mixed antagonist of oxytocin and vasopressin receptors [1].
Atosibana is a peptide and is a dual antagonist of oxytocin receptor (OTR) and vasopressin receptors (V1aR, V1bR, V2R). When acted as an OTR antagonist, atosibana showed pKi values of 7.9 and 7.2 for human OTR and rat OTR, respectively. Treatment of atosibana significantly inhibited cell growth in MDCK and HEK293 cells stably transfected with the human OTR with IC50 values of 15.4 nM and 20.8 nM, respectively. When acted as a vasopressin receptor antagonist, atosibana exerted pKi values of 9.8, 7.4 and 6.5 for hV1aR, hV1bR and hV2R, respectively. Atosibana has been shown to have efficacy in inhibiting uterine contractions and delaying preterm delivery. However, atosibana can not be used for long-term maintenance treatment since it is not orally bioavailable [1].
References:
[1] Borthwick A D. Oral oxytocin antagonists. Journal of medicinal chemistry, 2010, 53(18): 6525-6538.
[2] Reversi A, Rimoldi V, Marrocco T, et al. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. Journal of Biological Chemistry, 2005, 280(16): 16311-16318.
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















