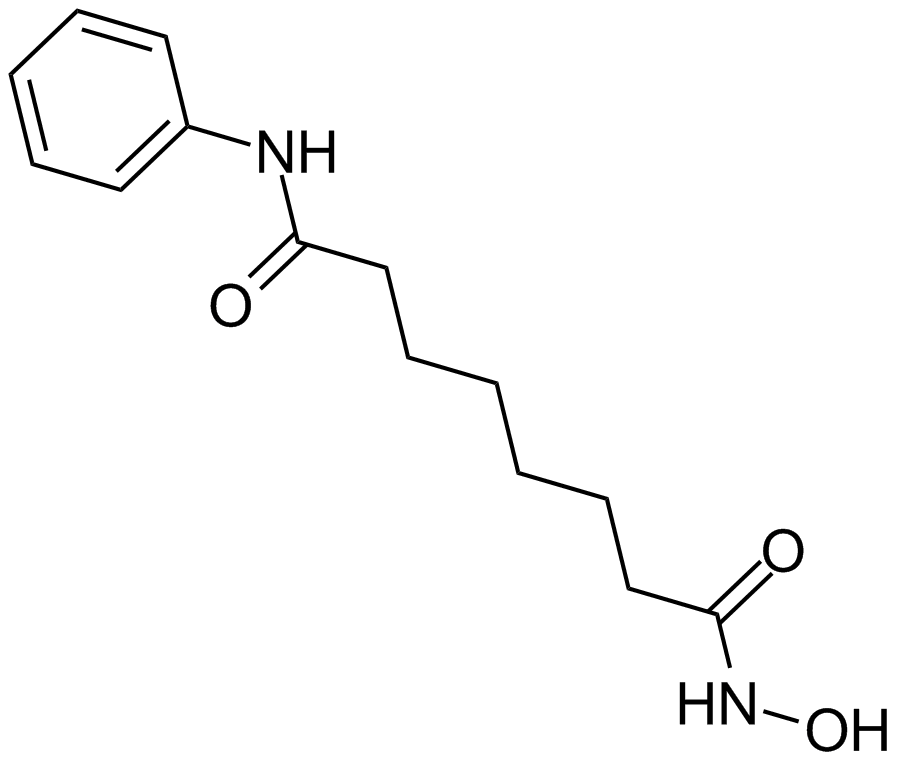
Vorinostat (SAHA, MK0683) (Synonyms: Suberoylanilide Hydroxamic Acid, Vorinostat) |
| Catalog No.GC17390 |
An HDAC inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 149647-78-9
Sample solution is provided at 25 µL, 10mM.
Vorinostat (suberoylanilide hydroxamic acid, SAHA) is a histone deacetylase inhibitor (HDACi), that plays key roles in epigenetic or non-epigenetic regulation, inducing growth arrest, differentiation and apoptosis of tumor cells.[1] Vorinostat is a small molecular with the formular of C14H20N2O3 and molecular weight of 264.3. The major mechanism of HDACi-induced apoptosis is the activation of the intrinsic apoptotic pathway. HDACi can activate the intrinsic apoptotic pathway by releasing of cytochrome c from mitochondria and regulating of Bcl-2 family expression.[2]
Reference
[1] Hui-ming Z, Qian-hai D, Wei-ping C, Ru-bin L. Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-kB nuclear translocation. International Immunopharmacology. 2013, 17. 329-335.
[2] Norihisa U, Sayaka K, Hisanori M, Katsuhiko Y, Airo T. Requirement of p38 MAPK for a cell-death pathway triggered by vorinostat in MDA-MB-231 human breast cancer cells. Cancer Letters. 2012, 315. 112-121.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




