β-Nicotinamide mononucleotide (Synonyms: βNMN) |
| Catalog No.GC16971 |
β-nicotinamide mononucleotide is a product of the nicotinamide phosphoribosyltransferase (NAMPT) reaction and a key NAD+ intermediate.
Products are for research use only. Not for human use. We do not sell to patients.
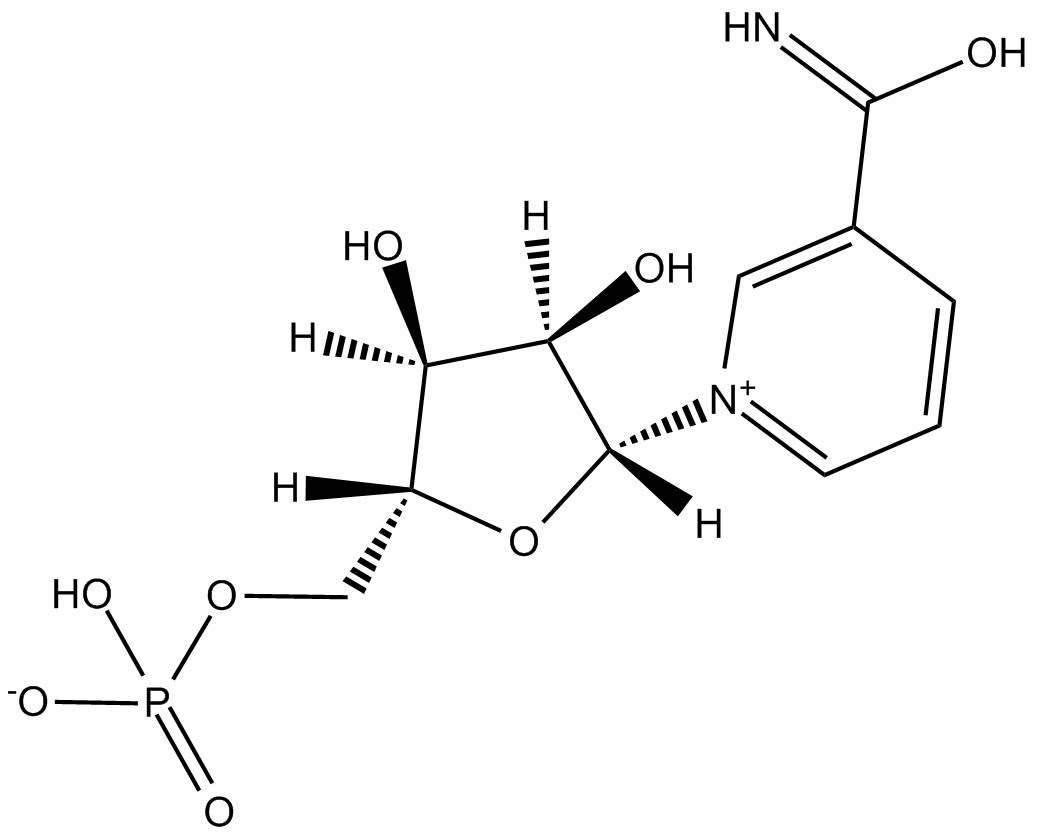
Cas No.: 1094-61-7
Sample solution is provided at 25 µL, 10mM.
β-nicotinamide mononucleotide is a product of the nicotinamide phosphoribosyltransferase (NAMPT) reaction and a key NAD+ intermediate. The pharmacological activities of β-nicotinamide mononucleotide include its role in cellular biochemical functions, cardioprotection, diabetes, Alzheimer's disease, and complications associated with obesity[1].
Intracellular NAD + levels were significantly reduced by knocking down or knocking down Nampt or treated with the Nampt inhibitor FK866, whereas NAD + levels were significantly increased by supplementation with NAD + precursors NAM or β-Nicotinamide mononucleotide [3].Treatment of β-nicotinamide mononucleotide, a precursor of NAD+, to HEK293 cells activated and improved the rate of mtDNA replication by increasing nucleotides in mitochondria and decreasing their degradation products: nucleosides. β-Nicotinamide mononucleotide metabolism plays a role in supporting mtDNA replication by maintaining the nucleotide pool balance in the mitochondria[7].
After β-Nicotinamide mononucleotide administration, there was no difference in blood glucose levels in GTTs between Nampt+/- and control female mice. In addition, β-Nicotinamide mononucleotide-treated Nampt+/- and control mice also had similar plasma insulin levels at each time point. β-Nicotinamide mononucleotide administration corrects the defect in GSIS observed in Nampt+/- mice[2]. β-nicotinamide mononucleotide ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice. β-nicotinamide mononucleotide also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation[4].In a mouse model induced by doxorubicin administered in divided low doses as in the clinics, supplementing mice with a precursor of NAD+ prevented the mtDNA depletion and cardiac dysfunction[5].When investigated whether β-Nicotinamide mononucleotide is superior to nicotinamide (Nam) as a precursor of NAD+ in whole animal experiments. β-Nicotinamide mononucleotide is retained in the body for longer than Nam[6].
References:
[1]: Poddar SK, Sifat AE, et,al. Nicotinamide Mononucleotide: Exploration of Diverse Therapeutic Applications of a Potential Molecule. Biomolecules. 2019 Jan 21;9(1):34. doi: 10.3390/biom9010034. PMID: 30669679; PMCID: PMC6359187.
[2]: Revollo JR, KÖrner A, et,al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007 Nov;6(5):363-75. doi: 10.1016/j.cmet.2007.09.003. PMID: 17983582; PMCID: PMC2098698.
[3]: Lv H, Lv G, et,al. NAD+ Metabolism Maintains Inducible PD-L1 Expression to Drive Tumor Immune Evasion. Cell Metab. 2021 Jan 5;33(1):110-127.e5. doi: 10.1016/j.cmet.2020.10.021. Epub 2020 Nov 9. PMID: 33171124.
[4]:Yoshino J, Mills KF, et,al. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011 Oct 5;14(4):528-36. doi: 10.1016/j.cmet.2011.08.014. PMID: 21982712; PMCID: PMC3204926.
[5]: Li J, Wang PY, et,al. p53 prevents doxorubicin cardiotoxicity independently of its prototypical tumor suppressor activities. Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19626-19634. doi: 10.1073/pnas.1904979116. Epub 2019 Sep 5. PMID: 31488712; PMCID: PMC6765288.
[6]: Kawamura T, Mori N, et,al. β-Nicotinamide Mononucleotide, an Anti-Aging Candidate Compound, Is Retained in the Body for Longer than Nicotinamide in Rats. J Nutr Sci Vitaminol (Tokyo). 2016;62(4):272-276. doi: 10.3177/jnsv.62.272. PMID: 27725413.
[7]:Cros C, Margier M, et,al. Nicotinamide Mononucleotide Administration Triggers Macrophages Reprogramming and Alleviates Inflammation During Sepsis Induced by Experimental Peritonitis. Front Mol Biosci. 2022 Jun 27;9:895028. doi: 10.3389/fmolb.2022.895028. PMID: 35832733; PMCID: PMC9271973.
Average Rating: 5 (Based on Reviews and 3 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




