Dehydroepiandrosterone (DHEA) (Synonyms: Androstenolone, trans-Dehydroandrosterone, DHEA, Diandrone, NSC 9896, Prasterone, Psicosterone) |
| Catalog No.GC11070 |
Dehydroepiandrosterone (DHEA) and its sulfate ester, DHEAS, together represent the most abundant steroid hormones in the human body .
Products are for research use only. Not for human use. We do not sell to patients.
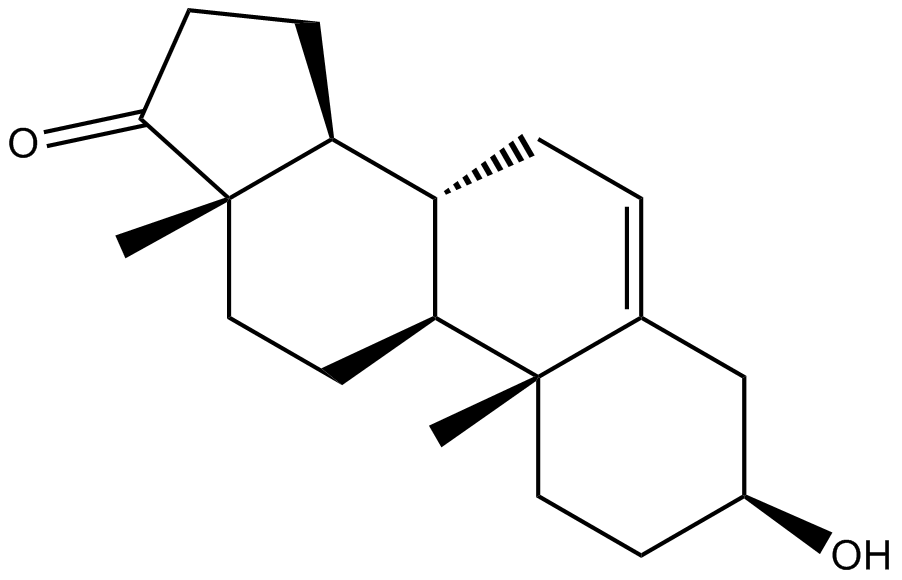
Cas No.: 53-43-0
Sample solution is provided at 25 µL, 10mM.
Dehydroepiandrosterone (DHEA) and its sulfate ester, DHEAS, together represent the most abundant steroid hormones in the human body [1].
Dehydroepiandrosterone (DHEA) (10-8 and 10-6 M) or DHEAS pretreated rat cerebral cortical cultures was increased neuronal survival when the cultures subjected to anoxia for 2 h [2]. When rat cerebral cortical cultures were subjected to anoxia for 2 h in an anaerobic chamber and pretreated with dehydroepiandrosterone (DHEA) (10-8 and 10-6 M) or DHEAS (10-6 M), there was increased neuronal survival. Using cultured neural precursors from rat embryonic forebrains, dehydroepiandrosterone (DHEA) (50 and 100 nM) activated the serine-threonine protein kinase Akt, which is widely implicated in cell survival signaling [3].
Dehydroepiandrosterone (DHEA) treating had better locomotor recovery on CD-1 female mice, after contusive spinal cord injury (SCI), and also left-right coordination, and fine motor control than control animals [4]. Mice treated with dehydroepiandrosterone (DHEA) also had significantly more white matter spared at the epicenter of the injury and reduced area of reactive gliosis surrounding the lesion. Dehydroepiandrosterone (DHEA) treatment was intensive and consisted of three different modes of administration: a DHEA Matrigel patch (10-10 M) applied to the spinal cord before closure of the wound, followed by 12 days of i.p. injections of saline containing Dehydroepiandrosterone (DHEA) (10-6 M or 0.02 mg/kg/day) after SCI, and Dehydroepiandrosterone (DHEA) (10-6 M) in the drinking water for 42 days [4].
References:
[1]. Rice SP, Zhang L, Grennan-Jones F, Agarwal N, Lewis MD, Rees DA, Ludgate M: Dehydroepiandrosterone (DHEA) treatment in vitro inhibits adipogenesis in human omental but not subcutaneous adipose tissue. Mol Cell Endocrinol 2010, 320: 51-57. 10.1016/j.mce.2010.02.017
[2]. C.E. Marx, L.F. Jarskog, J.M. Lauder, J.H. Gilmore, J.A. Lieberman, A.L. Morrow. Neurosteroid modulation of embryonic neuronal survival in vitro following anoxia. Brain Res., 871 (2000), pp. 104-112
[3]. L. Zhang, B. Li, W. Ma, J.L. Barker, Y.H. Chang, W. Zhao, D.R. Rubinow. Dehydroepiandrosterone (DHEA) and its sulfated derivative (DHEAS) regulate apoptosis during neurogenesis by triggering the Akt signaling pathway in opposing ways. Brain Res. Mol. Brain Res., 98 (2002), pp. 58-66
[4]. C. Fiore, D.M. Inman, S. Hirose, L.J. Noble, T. Igarashi, N.A. Compagnone. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J. Neurosci. Res., 75 (2004), pp. 391-400
Average Rating: 5 (Based on Reviews and 28 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















