Islatravir (MK-8591) (Synonyms: 4’Ed2FA, 4′-ethynyl-2-fluoro-2′-Deoxyadenosine, EFDA, MK-8591) |
| Catalog No.GC32306 |
Islatravir (MK-8591) (MK-8591) is a potent anti-HIV-1 agent, acting as a nucleoside reverse transcriptase inhibitor, with EC50s of 0.068 nM, 3.1 nM and 0.15 nM for HIV-1 (WT), HIV-1 (M184V), HIV-1 (MDR), respectively.
Products are for research use only. Not for human use. We do not sell to patients.
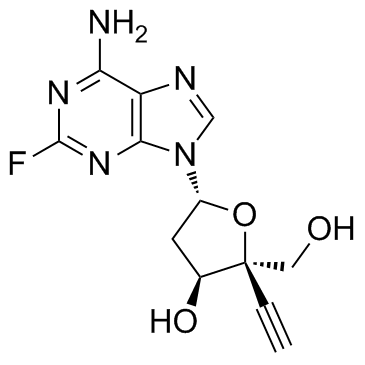
Cas No.: 865363-93-5
Sample solution is provided at 25 µL, 10mM.
Islatravir (MK-8591) is a potent anti-HIV-1 agent, acting as a nucleoside reverse transcriptase inhibitor, with EC50s of 0.068 nM, 3.1 nM and 0.15 nM for HIV-1 (WT), HIV-1 (M184V), HIV-1 (MDR), respectively.
Islatravir (MK-8591) (4'Ed2FA) is a potent anti-HIV-1 agent, acting as a nucleoside reverse transcriptase inhibitor, with EC50s of 0.068 nM, 3.1 nM and 0.15 nM for HIV-1 (WT), HIV-1 (M184V), HIV-1 (MDR), respectively[1].
[1]. Ohrui H, et al. 2'-deoxy-4'-C-ethynyl-2-fluoroadenosine, a nucleoside reverse transcriptase inhibitor, is highly potent against all human immunodeficiency viruses type 1 and has low toxicity. Chem Rec. 2006;6(3):133-43.
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















