NPPB (Synonyms: HOE 144, Hoechst 144) |
| Catalog No.GC14268 |
inhibitor of chloride channel
Products are for research use only. Not for human use. We do not sell to patients.
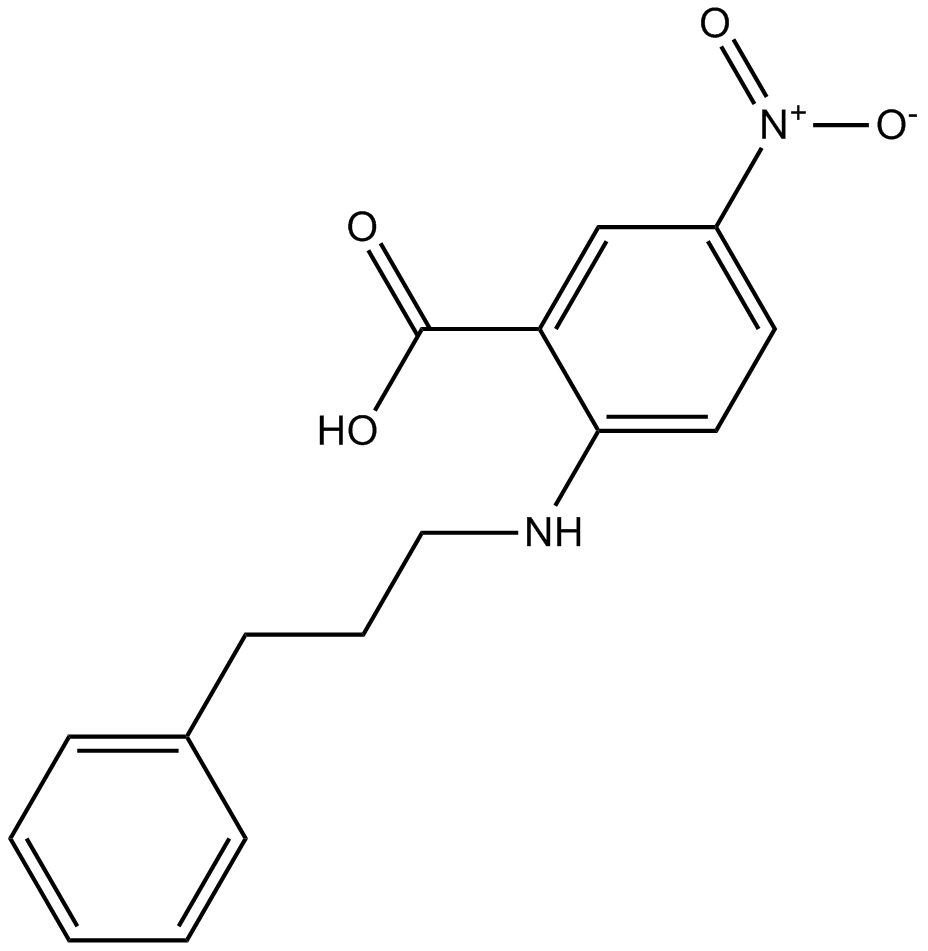
Cas No.: 107254-86-4
Sample solution is provided at 25 µL, 10mM.
NPPB, 5-nitro-2-(3-phenylpropylamino)-benzoic acid, is a potent inhibitor of chloride channel with IC50 of 80 nM for the short circuit current.[1]
Chloride channel blockers possess several sites of interaction, including the negatively charged carboxylate group, the secondary amine group which probably carries a positive partial charge, and for the very potent agents like NPPB an additional negative partial charge at the -NO2 substituent. In addition, an apolar interaction with a cycloaryl residue is necessary, and this site of interaction has a specific spacing from the secondary amino nitrogen.[1]
NPPB was evaluated for the activity on the equivalent short circuit current, corresponding to the secondary active transport of Cl- and measurements of the voltage across the basolateral membrane. The result revealed that NPPB possessed a good potency with IC50 of 80 nM for inhibiting the short circuit current. Furthermore, NPPB was also tested for its activity on various anion channels. Adopting freshly-isolated cells from the rat portal vein, the effects of NPPB were investigated on evoked and spontaneous currents by use of conventional whole-cell recording and perforated-patch techniques. At a holding potential of -60 mV in potassium-free, caesium-containing solutions, NPPB (10 μM) inhibited Ca-sensitive chloride currents (ICI(Ca)) evoked by caffeine (10 mM) and by noradrenaline (10μM) by the extend of 58% and 96%, respectively. In addition, at a holding potential of -2 mV in potassium -containing solutions, NPPB (10 μM) inhibited charybdotoxin-sensitive potassium-currents (IBK(Ca)) induced by noradrenaline (10 μM) and acetylcholine (10 μM) by approximately 90%. NPPB's inhibitory effects of volume-activated taurine, glucose, and uridine influxes was studied. The IC50 for the inhibition of the volume- activated fluxes by NPPB was around 12 μM. [1-3]
References:
[1] Wangemann, Ph, et al. "Cl−-channel blockers in the thick ascending limb of the loop of Henle Structure activity relationship." Pflügers Archiv 407.2 (1986): S128-S141.
[2] Kirkup, A. J., G. Edwards, and A. H. Weston. "Investigation of the effects of 5‐nitro‐2‐(3‐phenylpropylamino)‐benzoic acid (NPPB) on membrane currents in rat portal vein." British journal of pharmacology 117.1 (1996): 175-183.
[3] Kirk, Kiaran, J. C. Ellory, and J. D. Young. "Transport of organic substrates via a volume-activated channel." Journal of Biological Chemistry 267.33 (1992): 23475-23478.
Average Rating: 5 (Based on Reviews and 13 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




