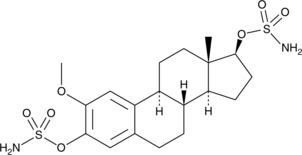
STX140 (Synonyms: 2-Methoxyestradiol-bis-sulphamate) |
| Catalog No.GC45688 |
An estrogen sulfamate
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 401600-86-0
Sample solution is provided at 25 µL, 10mM.
STX140 is an estrogen sulfamate with anticancer activities.1 It inhibits steroid sulfatase with IC50 values of 39 and 0.5 nM in placental microsomes and MCF-7 cancer cells, respectively. STX140 also binds to carbonic anhydrase IX and II (Kis = 70 and 270 nM, respectively).2 It inhibits bovine brain tubulin assembly in a cell-free assay (IC50 = 2.2 μM) and tubule formation in human umbilical vein epithelial cells (HUVECs) when used at concentrations of 50 and 100 nM.3,4 STX140 inhibits proliferation of LNCaP, PC3, and MDA-MB-231 cancer cells, as well as wild-type A2780 cancer cells and adriamycin- and cisplatin-resistant A2780 cancer cells (IC50s = 530, 400, 618, 330, 870, and 380 nM, respectively).5,6 It reduces angiogenesis in a Matrigel• plug assay in mice and tumor growth in MCF-7 and MDA-MB-231 mouse xenograft models when used at a dose of 20 mg/kg.7,6
|1. Raobaikady, B., Purohit, A., Chander, S.K., et al. Inhibition of MCF-7 breast cancer cell proliferation and in vivo steroid sulphatase activity by 2-methoxyoestradiol-bis-sulphamate. J. Steroid Biochem. Mol. Biol. 84(2-3), 351-358 (2003).|2. Andring, J.T., Dohle, W., Tu, C., et al. 3,17β-Bis-sulfamoyloxy-2-methoxyestra-1,3,5(10)-triene and nonsteroidal sulfamate derivatives inhibit carbonic anhydrase IX: Structure-activity optimization for isoform selectivity. J. Med. Chem. 62(4), 2202-2212 (2019).|3. Jourdan, F., Leese, M.P., Dohle, W., et al. Synthesis, antitubulin, and antiproliferative SAR of analogues of 2-methoxyestradiol-3,17-O,O-bis-sulfamate. J. Med. Chem. 53(7), 2942-2951 (2010).|4. Newman, S.P., Foster, P.A., Ho, Y.T., et al. The therapeutic potential of a series of orally bioavailable anti-angiogenic microtubule disruptors as therapy for hormone-independent prostate and breast cancers. Br. J. Cancer 97(12), 1673-1682 (2007).|5. Day, J.M., Newman, S.P., Comninos, A., et al. The effects of 2-substituted oestrogen sulphamates on the growth of prostate and ovarian cancer cells. J. Steroid Biochem. Mol. Biol. 84(2-3), 317-325 (2003).|6. Foster, P.A., Ho, Y.T., Newman, S.P., et al. 2-MeOE2bisMATE and 2-EtE2bisMATE induce cell cycle arrest and apoptosis in breast cancer xenografts as shown by a novel ex vivo technique. Breast Cancer Res. Treat. 111(2), 251-260 (2008).|7. Chander, S.K., Foster, P.A., Leese, M.P., et al. In vivo inhibition of angiogenesis by sulphamoylated derivatives of 2-methoxyoestradiol. Br. J. Cancer 96(9), 1368-1376 (2007).
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















