Temozolomide (Synonyms: CCRG 81045, MB 39831, Methazolastone, NSC 362856, Temodal, TMZ) |
| Catalog No.GC13667 |
Temozolomide is an oral activity alkylating agent that induces the formation of O6-methylguanine in DNA
Products are for research use only. Not for human use. We do not sell to patients.
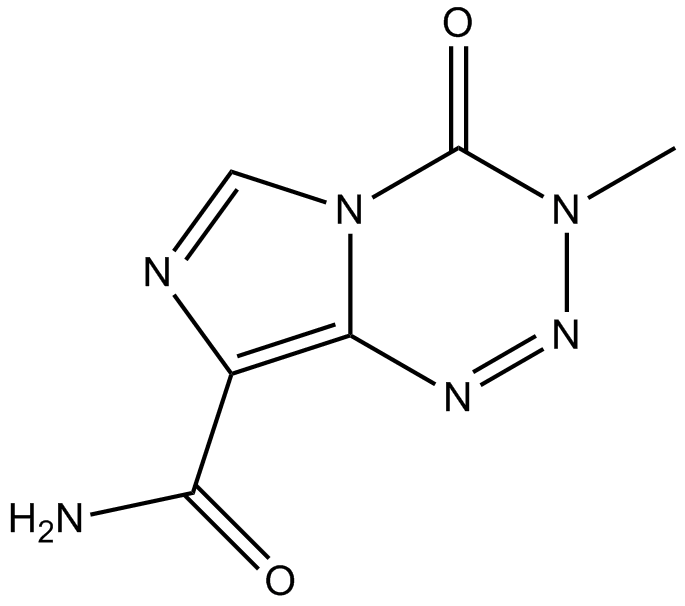
Cas No.: 85622-93-1
Sample solution is provided at 25 µL, 10mM.
Temozolomide is an oral activity alkylating agent that induces the formation of O6-methylguanine in DNA, which mispairs with thymine during the following cycle of DNA replication, leading to activation of the apoptotic pathways, Temozolomide could crosses the blood-brain barrier and is indicated for malignant gliomas and metastatic melanomas [1]. Temozolomide induced cell cycle arrest at G2/M and to eventually lead to apoptosis [2]. At physiologic pH it is converted to the short-lived active compound, MTIC. MTIC is further hydrolyzed to 5-amino-imidazole-4-carboxamide (AIC) and to methylhydrazine. The cytotoxicity of Temozolomide is mediated by its addition of methyl groups at N7 and O6 sites on guanines and the O3 site on adenines in genomic DNA. Alkylation of the O6 site on guanine leads to the insertion of a thymine instead of a cytosine opposite the methylguanine during subsequent DNA replication, and this can result in cell death [3].
Lymphoma cell counts performed 72 hours after treatment showed that the IC50 of Temozolomide was 44 µM (35-58 µM) when used alone and 16 µM (12-26 µM) when combined with NU1025 [1]. A time-related response in DNA strand-break formation was observed in the U251MG glioblastoma cells treated with Temozolomide (100 µM) alone [4]. Temozolomide was particularly evident when U-118 cells were incubated with Temozolomide concentrations of >100 µM. When U-118 cells were incubated with Temozolomide (250 µM) the proliferation rate was inhibited by 43.2% [5].
Temozolomide consistently demonstrates reproducible linear pharmacokinetics with approximately 100% p.o. bioavailability [6]. In the early stage s.c. implanted SNB-75 astrocytoma model, a 400-mg/kg dose of Temozolomide administered on Day 5 produced 10 of 10 Day 54 tumor-free mice. In later staged s.c. U251 and SF-295 glioblastoma models, a single 600-mg/kg dose produced 9 of 10 Day 86 and 2 of 10 Day 40 tumor-free mice, respectively. In the latter group, a tumor growth delay of >315% was attained [7]. Treatment of CEP 6800 (30 mg/kg) with Temozolomide (17 and 34 mg/kg) resulted in 100% complete regression of U251MG tumors by day 28 versus 60% complete regression caused by Temozolomide alone [4].
References:
[1]. Tentori L, Leonetti C, Scarsella M, et al. Combined treatment with temozolomide and poly (ADP-ribose) polymerase inhibitor enhances survival of mice bearing hematologic malignancy at the central nervous system site[J]. Blood, The Journal of the American Society of Hematology, 2002, 99(6): 2241-2244.
[2]. Baer J C, Freeman A A, Newlands E S, et al. Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells[J]. British journal of cancer, 1993, 67(6): 1299-1302.
[3]. Lee S Y. Temozolomide resistance in glioblastoma multiforme[J]. Genes & diseases, 2016, 3(3): 198-210.
[4]. Miknyoczki S J, Jones-Bolin S, Pritchard S, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly (ADP-ribose) polymerase inhibitor[J]. Molecular cancer therapeutics, 2003, 2(4): 371-382.
[5]. Carmo A, Carvalheiro H, Crespo I, et al. Effect of temozolomide on the U-118 glioma cell line[J]. Oncology letters, 2011, 2(6): 1165-1170.
[6]. Friedman H S, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma[J]. Clinical cancer research, 2000, 6(7): 2585-2597.
[7]. Plowman J, Waud W R, Koutsoukos A D, et al. Preclinical antitumor activity of temozolomide in mice: efficacy against human brain tumor xenografts and synergism with 1, 3-bis (2-chloroethyl)-1-nitrosourea[J]. Cancer research, 1994, 54(14): 3793-3799.
Average Rating: 5 (Based on Reviews and 8 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




