VX-809 (Synonyms: VX 809; VX809; Lumacaftor; VRT 826809) |
| Catalog No.GC15455 |
VX-809 (VX-809; VRT 826809) is a CFTR modulator that corrects the folding and trafficking of CFTR protein.
Products are for research use only. Not for human use. We do not sell to patients.
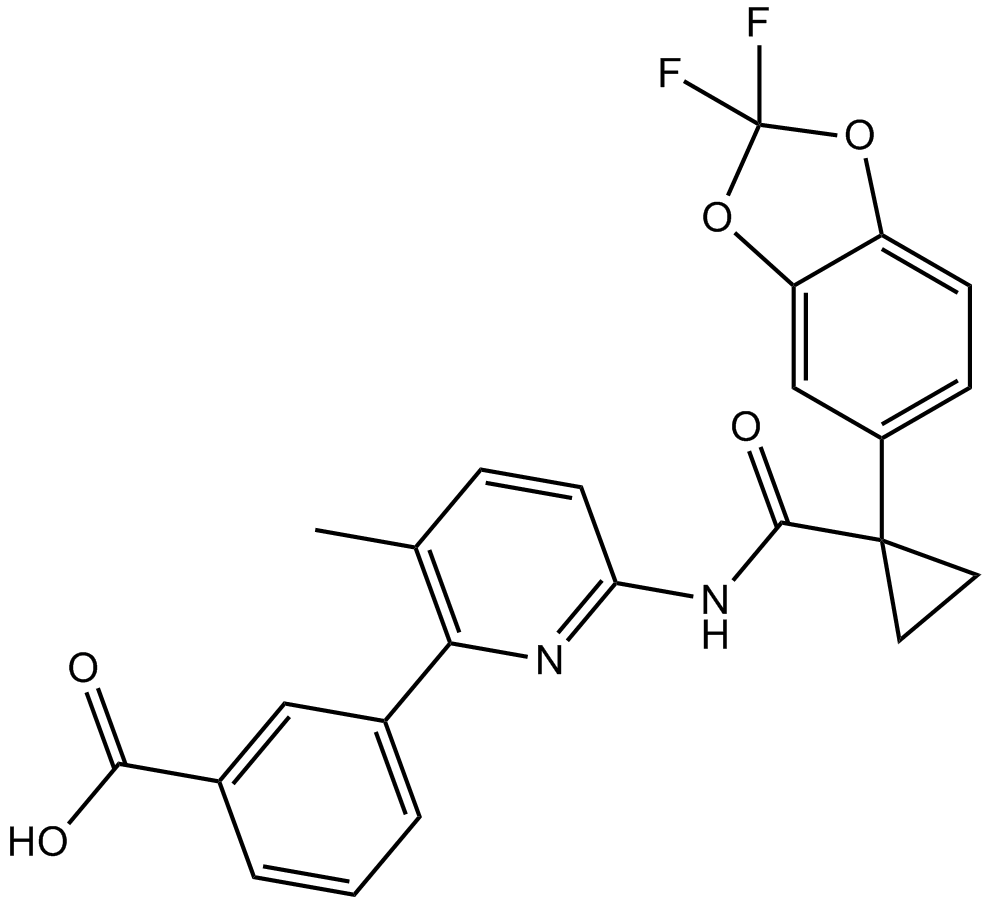
Cas No.: 936727-05-8
Sample solution is provided at 25 µL, 10mM.
VX-809 is a CFTR corrector that partially restores the function of F508del-CFTR. In Fischer rat thyroid (FRT) cells, it increases F508del-CFTR maturation at EC50 of 0.1 μM, and elevates F508del-CFTR–mediated chloride transport at EC50 of 0.5 μM [1]. It has no effect of other ion channels (hERG), transporter (P-gp) and disease-causing mislocalized proteins (α1-antitrypsin Z mutant) [1]. VX-809 stabilizes N-terminal fragment of CFTR that contain MSD1 by altering its protein conformation [2, 3].
Homozygous F508del-CFTR is the most common mutation in cystic fibrosis (CF) patients, accounting for 66–70% of CF cases worldwide. In cultured human bronchial epithelial cells that are homozygous for F508del, VX-809 restored the CFTR function and improved chloride and fluid Transport [1]. The combination of CFTR potentiators and VX-809 further improved the function of F508del-CFTR [4].
VX-809 has been tested in several clinical trials. Although it had minimal benefit to F508del-CFTR homozygous patients as a monotherapy [4], the result of Phase 2 study of VX-809 and KALYDECO combination showed significant improvements in lung function in CF patients with homozygous F508del-CFTR [5]. VX-809 is currently under investigation in two phase 3 trials.
References:
[1]Van Goor F, Hadida S, Grootenhuis PD et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011; 108: 18843-18848.[2] Ren HY, Grove DE, De La Rosa O et al. VX-809 corrects folding defects in cystic fibrosis transmembrane conductance regulator protein through action on membrane-spanning domain 1. Mol Biol Cell 2013; 24: 3016-3024.[3]Loo TW, Bartlett MC, Clarke DM. Corrector VX-809 stabilizes the first transmembrane domain of CFTR. Biochem Pharmacol 2013; 86: 612-619.[4]Clancy JP, Rowe SM, Accurso FJ et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 2012; 67: 12-18.[5]http://investors.vrtx.com/releasedetail.cfm?releaseid=687394
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















