Zinc Pyrithione |
| Catalog No.GC16453 |
Proton pump inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
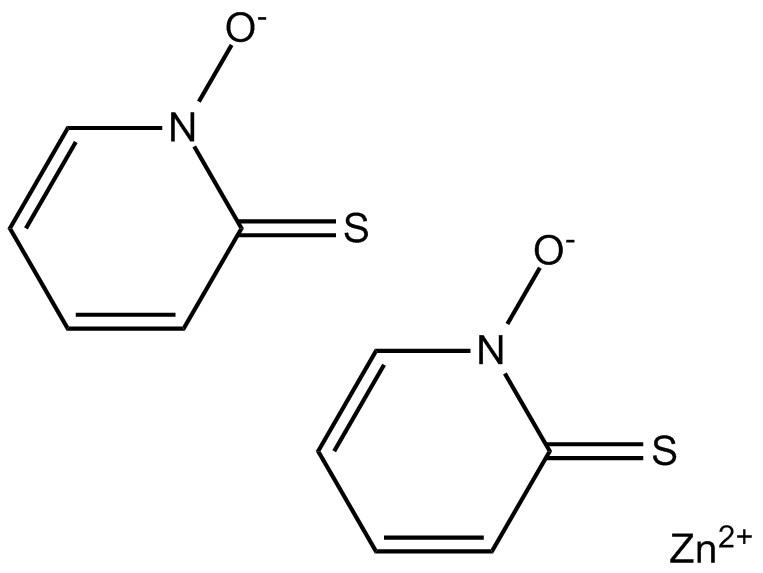
Cas No.: 13463-41-7
Sample solution is provided at 25 µL, 10mM.
Zinc Pyrithione is an antifungal and antibacterial agent disrupting membrane transport by blocking the proton pump.Target: Proton PumpZinc pyrithione is considered as a coordination complex of zinc. The pyrithione ligands, which are formally monoanions, are chelated to Zn 2+ via oxygen and sulfur centers. In the crystalline state, zinc pyrithione exists as a centrosymmetric dimer, where each zinc is bonded to two sulfur and three oxygen centers. In solution, however, the dimers dissociate via scission of one Zn-O bond. Zinc pyrithione, which is a dimer but is probably biologically active as a monomer, induces plasma membrane depolarization with half-maximal effect (K1/2) of about 0.3 mM [1]. Zinc pyrithione is an unusual synthetic potentiator that potently activates both heterologous and native M channels by inducing channel opening at the resting potential [2]. Zinc pyrithione rapidly accumulated in the tissues of the exposed mussels, proportionately to both exposure concentration and time. Even though the 7-d median lethal concentration (LC50) = 8.27 μM established here appears high with respect to reported ZnPT environmental concentrations, the results indicate that this biocide could represent a threat for marine organisms in coastal environments and that further investigations on its biological effects at sublethal doses are needed [3].
References:
[1]. Ermolayeva, E. and D. Sanders, Mechanism of pyrithione-induced membrane depolarization in Neurospora crassa. Appl Environ Microbiol, 1995. 61(9): p. 3385-90.
[2]. Xiong, Q., H. Sun, and M. Li, Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol, 2007. 3(5): p. 287-96.
[3]. Marcheselli, M., C. Rustichelli, and M. Mauri, Novel antifouling agent zinc pyrithione: determination, acute toxicity, and bioaccumulation in marine mussels (Mytilus galloprovincialis). Environ Toxicol Chem, 2010. 29(11): p. 2583-92.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















