Acyclovir (Synonyms: ACV, BW 248U, NSC 645011) |
| Catalog No.GC17186 |
Antiviral agent
Products are for research use only. Not for human use. We do not sell to patients.
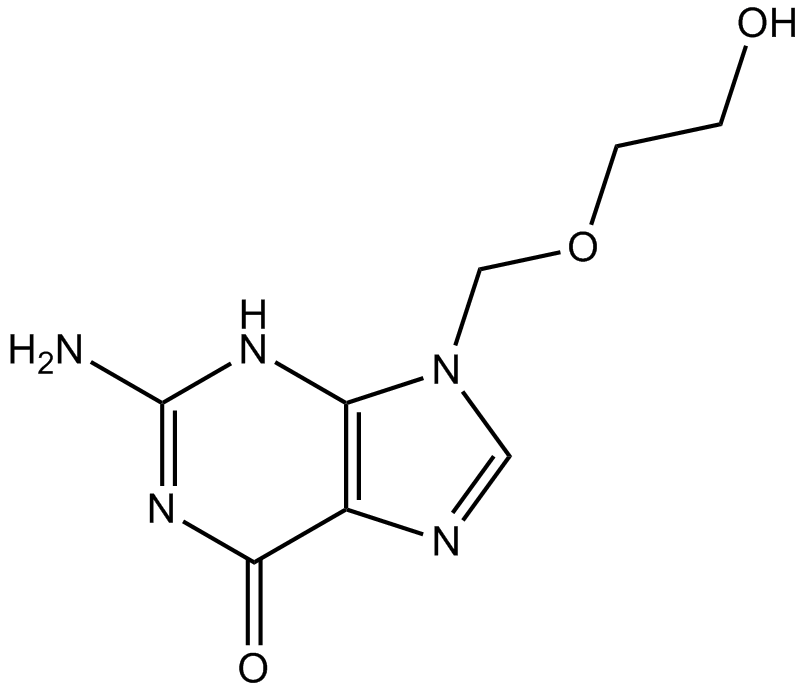
Cas No.: 59277-89-3
Sample solution is provided at 25 µL, 10mM.
Acyclovir, a molecule tailored to inactivate the thymidine kinase of the herpesvirus, is a guanosine analogue antiviral drug. It is a drug for HSV infection by GlaxoSmithKline.IC50 Value: 0.53-0.75 uM [3]Target: HSVin vitro: Acyclovir sensitivity was determined in a plaque-reduction assay in Vero cells. IC50 Values were consistently 2-3 fold lower in B2 compared with the H strain of Vero cells. HSV Type 2 strains were 2-10-fold less sensitive than Type 1 strains [2]. in vivo: two patients experienced a recurrence during treatment with oral acyclovir (200 mg 4 times daily) for up to 12 weeks, compared with nine during placebo treatment (P = 0.016). There was no difference between acyclovir and placebo in the time to the next recurrence following completion of treatment [3]. low-dose oral acyclovirmay be effective in the prevention of HSV infection during OKT3 treatment of seropositive patients. Continuation of acyclovir prophylaxis for two to four weeks following the conclusion of OKT3 therapy may prevent occurrence of delayed infections [4].Clinical trial: Acyclovir to Treat Patients Co-infected With HIV and Herpes Viruses in Uganda. Phage2
References:
[1]. Li Z, et al. Acyclovir treatment of skin lesions results in immune deviation in mice infected cutaneously with herpes simplex virus. Antivir Chem Chemother. 1999 Sep;10(5):251-7.
[2]. Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis. 1996 Apr;57(4):232-4.
[3]. Collins P, Oliver NM. Sensitivity monitoring of herpes simplex virus isolates from patients receiving acyclovir. J Antimicrob Chemother. 1986 Oct;18 Suppl B:103-12.
[4]. Meyrick Thomas RH, Dodd HJ, Yeo JM, Oral acyclovir in the suppression of recurrent non-genital herpes simplex virus infection. Br J Dermatol. 1985 Dec;113(6):731-5.
[5]. Tang IY, Maddux MS, Veremis SA, Low-dose oral acyclovir for prevention of herpes simplex virus infection during OKT3 therapy. Transplant Proc. 1989 Feb;21(1 Pt 2):1758-60.
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















