C646 |
| Catalog No.GC12733 |
C646, a potent and selective p300/CBP histone acetyltransferase inhibitor (Ki 400 nM), has been shown to have pleiotropic activity, including neuroprotective, anti-cancer and anti-epithelial-mesenchymal transition (anti-EMT) effects..
Products are for research use only. Not for human use. We do not sell to patients.
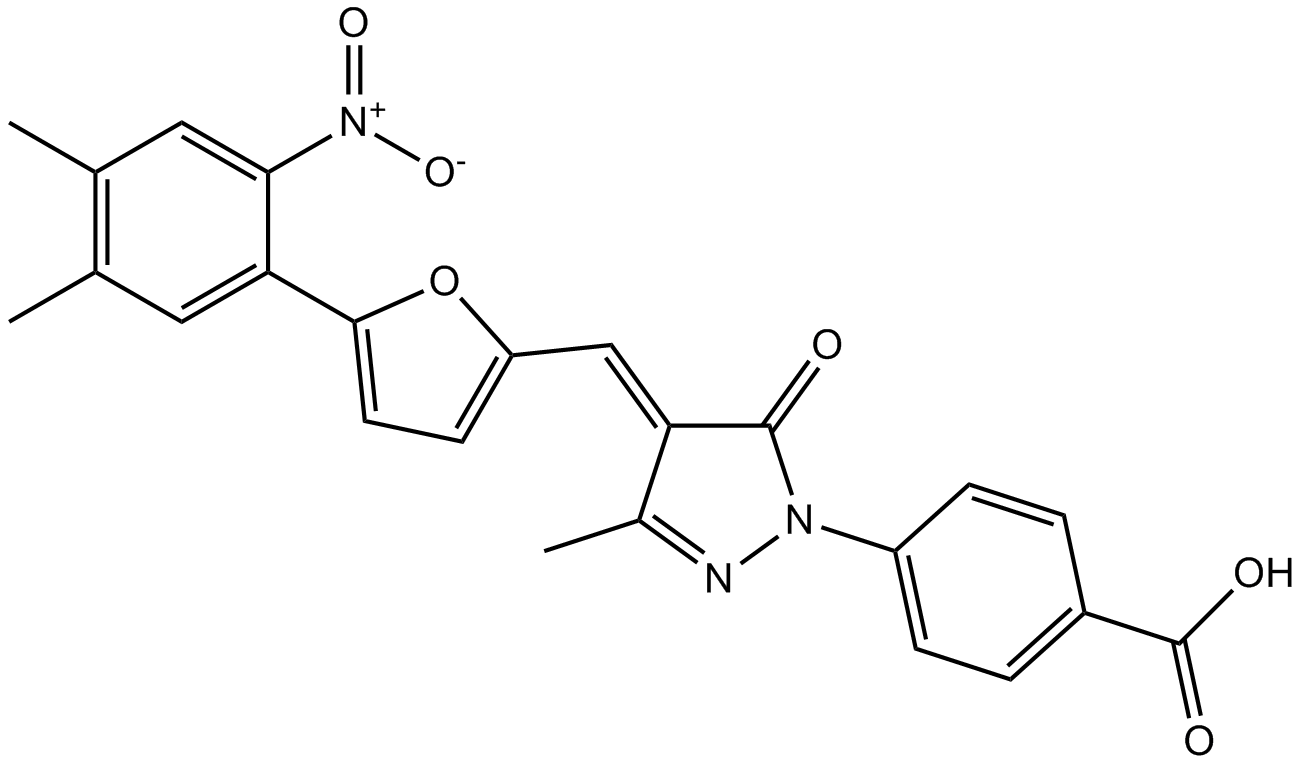
Cas No.: 328968-36-1
Sample solution is provided at 25 µL, 10mM.
C646, a potent and selective p300/CBP histone acetyltransferase inhibitor (Ki 400 nM), has been shown to have pleiotropic activity, including neuroprotective, anti-cancer and anti-epithelial-mesenchymal transition (anti-EMT) effects[1-4].
C646(10µM; 2 h) suppresses LPS-stimulated cytokines production[5]. C646(10-50 µM) inhibits histone H3 acetylation and proliferation of pancreatic cancer cells[6]. C646(20 µM; 4 h) activates insulin signaling and increases insulin receptor protein substrates(IRS) membrane translocation in the absence of insulin in Hepa1-6 cells[7].
C646(10 ul; one dose per day; 7days) attenuated mechanical allodynia and thermal hyperalgesia, accompanied by a suppressed COX-2 expression in the spinal cord[8]. C646(1 µg/gm; i.v;14days) treatment attenuated p300 and H3K56 acetylation and improved arterial stiffness and Coronary flow reserve (CFR) via improvement of endothelial cell (EC) dysfunction and suppression of NF-κB[9].
References:
[1]. Oike T, Komachi M, et,al. C646, a selective small molecule inhibitor of histone acetyltransferase p300, radiosensitizes lung cancer cells by enhancing mitotic catastrophe. Radiother Oncol. 2014 May;111(2):222-7. doi: 10.1016/j.radonc.2014.03.015. Epub 2014 Apr 17. PMID: 24746574.
[2]. Yang Y, Liu K, et,al. Histone acetyltransferase inhibitor C646 reverses epithelial to mesenchymal transition of human peritoneal mesothelial cells via blocking TGF-β1/Smad3 signaling pathway in vitro. Int J Clin Exp Pathol. 2015 Mar 1;8(3):2746-54. PMID: 26045780; PMCID: PMC4440089.
[3]. Zhu XY, Huang CS, et,al. p300 exerts an epigenetic role in chronic neuropathic pain through its acetyltransferase activity in rats following chronic constriction injury (CCI). Mol Pain. 2012 Nov 23;8:84. doi: 10.1186/1744-8069-8-84. PMID: 23176208; PMCID: PMC3558366.
[4]. Bowers EM, Yan G, et,al. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem Biol. 2010 May 28;17(5):471-82. doi: 10.1016/j.chembiol.2010.03.006. PMID: 20534345; PMCID: PMC2884008.
[5]. Fang F, Li G, et,al. C646 modulates inflammatory response and antibacterial activity of macrophage. Int Immunopharmacol. 2019 Sep;74:105736. doi: 10.1016/j.intimp.2019.105736. Epub 2019 Jul 11. PMID: 31302452.
[6]. Ono H, Kato T, et,al. C646 inhibits G2/M cell cycle-related proteins and potentiates anti-tumor effects in pancreatic cancer. Sci Rep. 2021 May 12;11(1):10078. doi: 10.1038/s41598-021-89530-8. PMID: 33980911; PMCID: PMC8115044.
[7]. Peng J, Ramatchandirin B, et,al. The P300 acetyltransferase inhibitor C646 promotes membrane translocation of insulin receptor protein substrate and interaction with the insulin receptor. J Biol Chem. 2022 Mar;298(3):101621. doi: 10.1016/j.jbc.2022.101621. Epub 2022 Jan 21. PMID: 35074429; PMCID: PMC8850660.
[8]. Zhu XY, Huang CS, et,al. p300 exerts an epigenetic role in chronic neuropathic pain through its acetyltransferase activity in rats following chronic constriction injury (CCI). Mol Pain. 2012 Nov 23;8:84. doi: 10.1186/1744-8069-8-84. PMID: 23176208; PMCID: PMC3558366.
[9]. Su H, Zeng H, et,al. Histone Acetyltransferase p300 Inhibitor Improves Coronary Flow Reserve in SIRT3 (Sirtuin 3) Knockout Mice. J Am Heart Assoc. 2020 Sep 15;9(18):e017176. doi: 10.1161/JAHA.120.017176. Epub 2020 Aug 31. PMID: 32865093; PMCID: PMC7727016.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















