E-64 |
| Catalog No.GC13418 |
A fungal metabolite with diverse biological activities
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 66701-25-5
Sample solution is provided at 25 µL, 10mM.
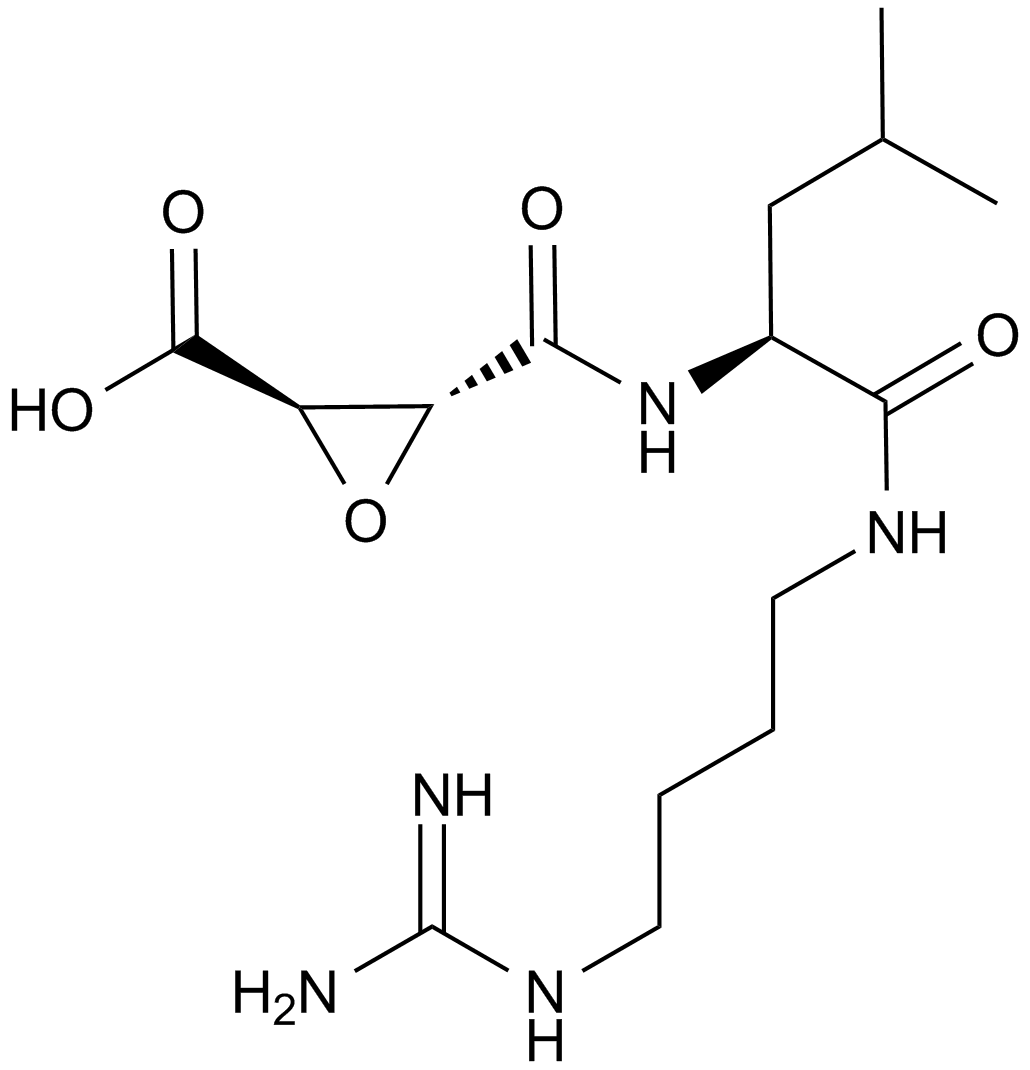
A new class of compounds that show promise of acting as class-specific inhibitors for the cysteine proteinases are the L-trans-epoxysuccinylpeptides related to the compound E-64 [L-trans-epoxysuccinyl-L-leucylamido(4-guanidino)butanel , isolated from cultures of Aspergillus. E-64 was shown to inhibit papain, ficin and the fruit and stem bromelains, with disappearance of the thiol group of papain1.
E-64 has been reported to inhibit two other mammalian cysteine proteinases: cathepsin L3 and a proteinase from human breast-tumour tissue4 and the calcium-dependent proteinase, calpain, from chicken muscle5. All of these characteristics suggested that E-64 might be a valuable inhibitor for the study of cysteine proteinases.
Lineweaver-Burk plots of inhibition data show that the action of E-64 was not competitive with substrate1 . Moreover, the optical isomerism of the epoxysuccinyl moiety seemed to have no effect on the activity of E-64 as an inhibitor of papain6, 7 .If E-64 were indeed acting by covalent reaction at the active site, its rate of reaction would be decreased by the presence of leupeptin, a tight-binding reversible inhibitor8.
E-64 inhibits only cysteine proteinases. Papain showed a particularly high reactivity with E-64, and good rates were also obtained with the other plant enzymes and the lysosomal cysteine proteinases. There is structural evidence that these enzymes form a homologous group9, and they resemble each other in having Mr about 25 000, no (detected) zymogens and no distinct requirement for calcium. Chicken skeletal-muscle calpain is reported to be inhibited by E-64, but the rate constant has not been determined5.
The most obvious practical application of E-64 is in the active-site titration of the papain-related cysteine proteinases. Active-site titration as a method of determining enzyme concentration has the advantage over rate assays of being insensitive to reaction conditions, and giving a result in active-site molarity10 (Bender et al., 1966).
References:
1. A. J. BARRETT, A. A. KEMBHAVI, L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem. J. (1982) 201, 189-198
2. Hanada, K., Tamai, M., Yamagishi, M., Ohmura, S., Sawada, J. & Tanaka, I. (1978c) Agric. Biol. Chem. 42, 523-528
3. Towatari, T., Tanaka, K., Yoshikawa, D. & Katunuma, N. (1978).J. Biochem. (Tokyo) 84, 659-671.
4. Mort, J. S., Recklies, A. D. & Poole, A. R. (1980) Biochim. Biophys. Acta 614, 134-143.
5. Sugita, H., Ishiura, S., Suzuki, K. & Imahori, K. (1980) J. Biochem. (Tokyo) 87, 339-341
6. Hanada, K., Tamai, M., Morimoto, S., Adachi, T.,Ohmura, S., Sawada, J. & Tanaka, I. (1978a) Agric. Biol. Chem. 42, 537-541.
7. Hanada, K., Tamai, M., Ohmura, S., Sawada, J., Seki, T.& Tanaka, I. (1978b)Agric. Biol. Chem. 42, 529-536
8. Knight, C. G. (1980) Biochem. J. 189,447-453
9. Takio, K., Towatari, T., Katunuma, N. & Titani, K.(1980) Biochem. Biophys. Res. Commun. 97, 340-346
10. Bender, M. L., Begue-Canton, M. L., Blakeley, R. L.,Brubacher, L. J., Feder, J., Gunter, C. R., Kezdy, F. J.,Killheffer, J. V., Marshall, T. H., Miller, C. G., Roeske,R. W. & Stoops, J. K. (1966) J. Am. Chem. Soc. 88,5890-5913
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















