Etoposide (Synonyms: VP-16; VP-16-213) |
| Catalog No.GC15617 |
Topo II inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
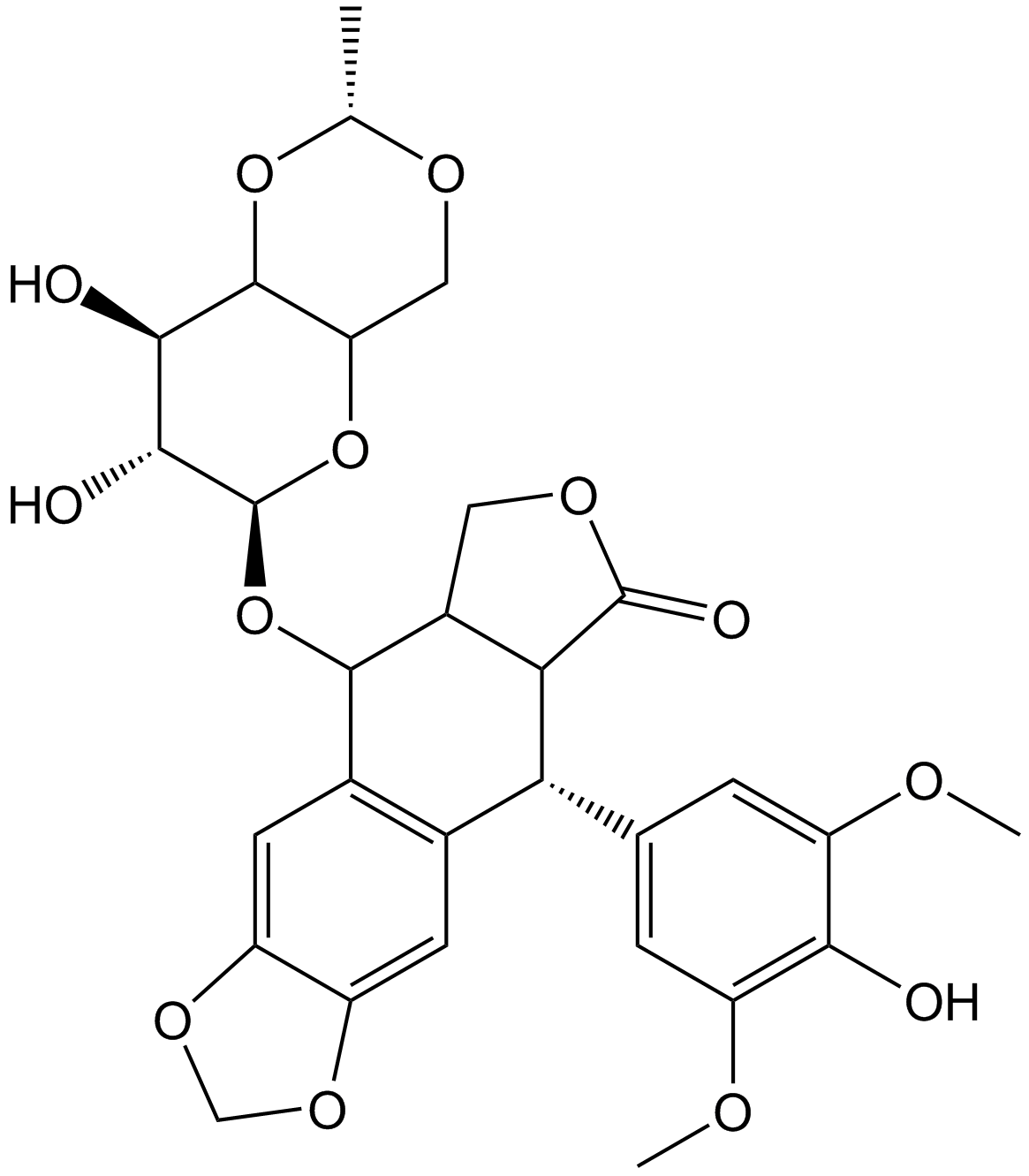
Cas No.: 33419-42-0
Sample solution is provided at 25 µL, 10mM.
Etoposide (VP-16) is the first agent recognized as a topoisomerase II inhibitor of anticancer drug with IC50 of 59.2 μM.
The activity of the topoisomerase II enzyme on re-ligation of DNA strands is interrupted by etoposide. A ternary complex with DNA is formed by etoposide, and causes DNA strands to break [1]. The enzyme was more important in cancer cell than healthy cells, because cancer cells divided more rapidly. So etoposide induced apoptosis of the cancer cells [2]. Etoposide exhibited cytotoxic activity against HepG2 and MOLT-3 cancer cells with IC50 of 30.16 μM and 0.051μM [3]. The IC50 values of etoposide against the tumor cell lines of BGC-823, HeLa, and A549 were 43.74 ± 5.13, 209.90 ± 13.42, and 139.54 ± 7.05 μM, respectively [4].
References:
[1]. Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010 May 28;17(5):421-33.
[2]. Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA. Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon. 2004 Sep 15;44(4):441-59.
[3]. Pingaew R, Mandi P, Nantasenamat C, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. Design, synthesis and molecular docking studies of novel N-benzenesulfonyl-1,2,3,4-tetrahydroisoquinoline-based triazoles with potential anticancer activity. Eur J Med Chem. 2014 May 6;81C:192-203.
[4]. Xiao L, Zhao W, Li HM, Wan DJ, Li DS, Chen T, Tang YJ. Design and synthesis of the novel DNA topoisomerase II inhibitors: Esterification and amination substituted 4'-demethylepipodophyllotoxin derivates exhibiting anti-tumor activity by activating ATM/ATR signaling pathways. Eur J Med Chem. 2014 Jun 10;80:267-77.
Average Rating: 5 (Based on Reviews and 15 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















